3814
Quantification of MRI visibility and artefacts at 3T of liquid fiducial marker in a pancreas tissue mimicking phantomSergej Schneider1,2, Rasmus Irming Jølck3,4, Esther Gera Cornelia Troost1,2,5,6,7, and Aswin Louis Hoffmann1,2,5
1Institute of Radiooncology-OncoRay, Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany, 2OncoRay – National Center for Radiation Research in Oncology, Faculty of Medicine and University Hospital Carl Gustav Carus, Technische Universität Dresden, Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany, 3Nanovi Radiotherapy A/S, Kgs. Lyngby, Denmark, 4Department of Micro- and Nanotechnology, Center for Nanomedicine and Theranostics, Technical University of Denmark, Kgs. Lyngby, Denmark, 5Department of Radiotherapy and Radiation Oncology, Faculty of Medicine and University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany, 6German Cancer Consortium (DKTK), partner site Dresden, and German Cancer Research Center (DKFZ), Heidelberg, Germany, 7National Center for Tumor Diseases (NCT), partner site Dresden, Dresden, Germany
Synopsis
In image-guided radiotherapy (IGRT) of patients with pancreatic ductal adenocarcinoma (PDAC), implanted fiducial gold markers are used for position verification and for registration of computed tomography (CT) and MRI scans used for delineation purposes. Recently, a liquid biodegradable carbohydrate based injectable soft tissue marker, compatible with CT and MRI, has been developed. Our aim was to quantitatively evaluate the tradeoff between visibility and artifacts of this marker on MRI and compare this with two solid fiducial gold markers commonly used in IGRT of PDAC.
Introduction
Image-guided radiotherapy (IGRT) of pancreatic ductal adenocarcinoma (PDAC) based on implanted fiducial markers and daily orthogonal kV X-ray imaging or cone-beam computed tomography (CBCT) has been shown to significantly reduce the setup error as compared to bony alignment1. In state-of-the-art IGRT solid gold markers are implanted into the pancreas using endoscopic ultrasonography (EUS), a procedure that is well established and generally well tolerated. However, solid gold markers not only deteriorate image quality in both CT and MRI, but additionally cause significant dose alterations in particle therapy, showing local dose perturbations up to 80% of the prescribed dose. Recently, a new biodegradable liquid marker has been developed, which forms a gel-like marker after injection into soft tissue. This marker may particularly benefit patients with PDAC who are scheduled for particle therapy, because it can be implanted using very thin (≤25 G) needles, its low Z-elemental (non-ferrous and non-magnetic) composition causes minimal proton dose perturbation in soft tissues, its size and visibility on X-ray images, CT and CBCT can be adjusted by controlling the injected volume and its soft-surface adhesiveness may decrease migration behaviour relative to solid markers. So far, the characteristics of the liquid marker on magnetic resonance imaging (MRI) have not been investigated. It is the aim of the present work to provide a quantitative, pulse sequence-independent assessment of the visibility and artefacts of the new liquid fiducial marker on MRI and compare them against those of two gold markers commonly applied in IGRT of PDAC.Methods
To quantify the propensity of the different markers to generate signal voids and signal shifts on MRI, a spherical gel phantom mimicking the relaxation properties of healthy pancreatic tissue at 3 Tesla was constructed. Different volumes (10 µL, 25 µL, 50 µL and 100 µL) of the liquid marker (BioXmark®, Nanovi A/S) were casted into the gel as well as four Gold Anchor™ (Naslund Medical AB; 0.28 mm diameter, 10 mm and 20 mm length) and three VisiCoil™ (IBA Dosimetry; 0.35 mm diameter, 5 mm and 10 mm length) markers, implanted in different orientations. MR relaxometry was performed to quantify the size and magnitude of the decrease in the effective transversal relaxation time T2* and water proton density ρ(H) relative to pure water as a measure of potential visibility, and to quantify the size and magnitude of the increase in magnetic field inhomogeneity ΔB0 as a measure of potential signal artefacts. The phantom was scanned with a 3.0 T Philips Ingenuity TF PET/MR scanner using an 8-channel head coil.Results
The solid fiducial markers showed a direct linear relationship between the potentially visible size and artefact size. The liquid fiducial marker showed a tendency towards a potentially visible size at smaller artefacts. Liquid markers from 25-100 μL generated visible volumes comparable to the visible size of the solid markers. The visible magnitude was the largest for the liquid fiducial marker with volumes of 25μL – 100μL showing no correlation with the magnitude of artefact. The solid markers showed a strong non-linear correlation between magnitude of visibility and artefact. The gold-iron alloy marker induced the strongest artefacts.Discussion
The liquid fiducial marker causes signal voids on MRI due to its absence of water hydrogen atoms without strongly affecting the magnetic field in the surrounding tissue. The alteration of the static magnetic field was found to be the main effect leading to the visibility of the solid fiducial markers.Conclusion
The liquid fiducial marker has beneficial MRI properties regarding the trade-off between potential visibility and artefacts compared to the tested solid gold markers that are currently being used for IGRT of PDAC. Contrary to the solid markers, an increase in visibility of the liquid marker was not directly coupled to an increase in artefact. Due to the proton density reduction effect, the liquid marker behaves comparably in all pulse sequences if acquired at similar resolution.Acknowledgements
The authors thank G. Lymperopoulou (IBA Dosimetry GmbH) for providing the VisiCoil™ and Dr. I. Näslund (Naslund Medical AB) for providing the Gold Anchor™ markers. Furthermore we are grateful to T. Jepsen (Nanovi A/S) for providing BioXmark® markers and for the support in the construction of the phantom. Finally we express our gratitude to Dr. B. Mädler (Philips GmbH) for support and advice on technical aspects of MR image acquisition and processing.References
1. Van der Horst A, Wognun S, Fajardo RD et al. Interfractional position variation of pancreatic tumors quantified using intratumoral fiducial markers and daily cone beam computed tomography. IJROBP 2013; 87, 202-8Figures

Figure 1. (a) Spherical case of the MRI-compatible pancreas-equivalent gel phantom
positioned on a cork ring for stability. (b) Coronal T1-weighted MR image of the central plane in
which all 11 fiducial markers were
implanted. Four Gold Anchor™ markers can be seen in the bottom, four BioXmark® are aligned in the center and three
VisiCoil™ markers were placed
in the top. (c) Transversal T1-weighted MR image,
showing the sphere containing MQ-H2O at the bottom and four
BioXmark® at the top.
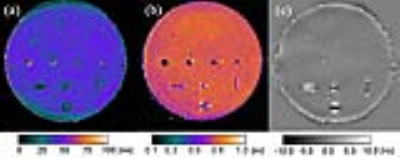
Figure 2. Central coronal plane
of the phantom showing (a) the T2* map, (b) the ρ(H)-map in percent units
[pu](fraction of ρ(H) of investigated voxel relative to ρ(H) in water), (c) the with a Gaussian-filter subtracted
ΔB0-map.
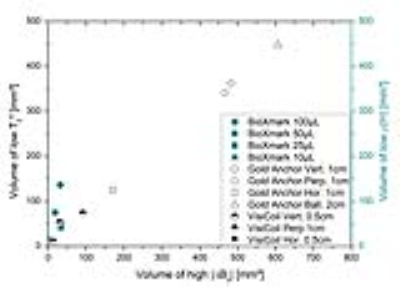
Figure 3. Trade-off between the
volume with low T2* relaxation time (left axis) and low proton density ρ(H)
(right axis) as a measure of potentially visible size and the volume with high
ΔB0 as a measure of potential size of artefact.

Figure 4. Maximum reduction of transverse relaxation time T2*
(left axis) and relative proton density p(H) (right axis) in percent units [pu]
(as a measure of potentially visible magnitude) as function of the maximum
magnetic field inhomogeneity introduced by the marker (as a measure of magnitude
of potential artefacts).