3807
4D-MRI in radiotherapy: motion phantom study and automatic sorting based on internal surrogate for motion management during free breathing1Department of Engineering and Medical Physics, Institut Universitaire du Cancer de Toulouse - Oncopole, Toulouse, France, 2Department of Medical Physics, University of Basel, Basel, Switzerland, 3Department of Biomedical Engineering, University of Basel, Basel, Switzerland
Synopsis
4D-MRI sequence for radiotherapy (RT) application is validated in this study on a motion phantom and on healthy volunteers. 4D-MR imaging of a moving spherical target with different signal waveforms gave similar results as for classical 4D-CT used in RT planning. During free breathing 4D-MRI acquisitions, the internal surrogates, defined as the mean intensity inside a region of interest are relevant to detect the different respiratory phases for consistent retrospective automatic image sorting. Feasibility of integration into treatment planning system was also demonstrated, allowing picturing the dynamic behavior of the moving organ for treatment planning.
Introduction
MR abdominal motion imaging is handled at our institute in clinical routine with acquisitions triggered on exhale phase. MR images are registered with 4D-CT for tumor delineation as better soft tissue contrast is given by MR modality. However in this configuration, only the exhale phase of the respiratory cycle is registered. Therefore the dynamic behavior and the organ deformation are not captured. This study aims at presenting a novel retrospective gating approach for dynamic MR imaging on a motion phantom and during free breathing.Material& Method
Motion phantom study
Various motion waveforms are applied to a 3.3cm diameter sphere using the QuasarTM MRI4D motion phantom (Modus QA, London, ON, Canada). Respiratory cycles are configured with different sinusoidal waves of 4, 5 and 6 s period and 10, 20 and 30 mm peak-to-peak amplitude. The phantom is first imaged on a 1.5T MR scan using a 18-channel body flex coil. The 4D-MRI innovative sequence is based on a modified bSSFP sequence [1-2] and consists of an interleaved acquisition of 2D image slices and navigators. The navigator is set and acquired at a fixed position in sagittal orientation, while axial image position is changing in order to cover the entire volume of interest [3]. 4D-MR images are acquired over 40 slices with a 2.5mm thickness, TE/TR=2.43/440.75ms, 1.04x1.04mm2 resolution and a 0.44ms slice-time-resolution. The sequence is repeated 10 times to cover entire respiratory cycles.
For comparison with a
conventional RT technique, the same phantom is imaged on our 4D-CT scan.
Quantitative evaluation consisted of comparing against the actual values the
sphere volume on each phase of both 4D-MR and 4D-CT image sets as well as the
sphere displacement amplitude.
Volunteers study
4D-MRI acquisitions with the same protocol as for the motion phantom (with 20 repetitions) are performed on healthy volunteers who gave their informed consent. Audio-coaching in the MR scan is provided and volunteers are asked to breathe at a constant period of 6 sec. Total acquisition time is less than 12min.
For 4D-MR images
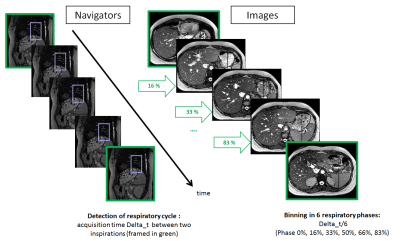
reconstruction, the period of the respiratory cycle is divided in 6 bins: 0%,
16%, 33%, 50%, 66% and 83%. This strategy is copied from 4D-CT, in order to
prepare consistent registration for accurate lesion contouring. Motion position
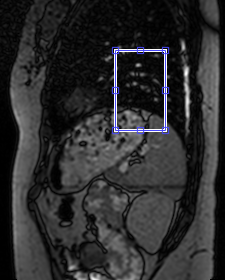
is given by the mean intensity in a rectangular region of interest (ROI) placed
at the level of the diaphragm on the sagittal navigator (Fig.1).
Results
Motion phantom study
The sphere volume is overestimated on MRI: for a given wave amplitude of 20mm, the sphere volume measured on MRI increases with breathing period (from 7 to 29%). Similar effects are observed on CT images. For a given breathing period of 6s, the sphere volume measured on MRI increases with wave amplitude. This effect is less pronounced on CT images. The sphere displacement is underestimated with both modalities (up to 20%). For a given wave amplitude of 20mm, the displacement amplitude is better estimated on MRI for the smallest period of 4s whereas CT gives the worst estimate for this period. For a given breathing period of 6s, the displacement amplitude is underestimated on MRI by 20 % whatever the peak-to-peak amplitude, while on CT the worst estimate is obtained for the largest amplitude.
Volunteers study
Mean intensity time course inside the ROI is able to identify automatically the inspiration positions (phase 0%). Acquisition time interval between two consecutive inspirations defines each experimental respiratory cycle for the 20 repeated MR acquisitions.MR image slices are automatically binned according to the 6 respiratory phases and stacked in 3D volumes (Fig.2). As the ROI is defined by the user, 5 ROI voluntarily positioned differently, but still on the diaphragm, were tested. This method is not sensitive to the ROI position as inspiration phases were reproducibly detected. Along the repeated acquisitions, mean respiratory cycles is 5.68 sec [1.76-7.92]. For the same navigator slices, 94.5% of the inspiration positions are found each time. The volunteer for whom these results are given recognizes her respiration wasn't always synchronized with the audio-coaching.
Conclusion
The motion phantom study showed that this new 4D-MR sequence succeeded in imaging a moving target with similar performances as a conventional 4D-CT. The overestimation of sphere volume on MR images is not specific of the 4D-MR sequence as it was also observed on static images. The study on volunteers confirms that retrospective automatic sorting of 4D-MR images acquired during free breathing is possible with a simple rectangular ROI, used as internal surrogate, placed on navigator slice. Future work will consist on measurements of patients with gold fiducials and eligible for liver stereotactic body RT, in order to capture the entire dynamic behavior of the liver, fiducials and lesion.Acknowledgements
The authors are thankful to Karen Mkhitaryan (Siemens Healthcare) for his help in the set-up of MR sequence and the MODUS QA team for their help with motion phantom.References
[1] M.von Siebenthal et al, Phy.Med.Biol. 2007
[2] Z.Celicanin et al, Magn Reson.Med. 2015
[3] S.Ken et al, MReadings: MR in RT Magnetom 2017
Figures