3800
Lonidamine-induced selective acidification and deenergization of prostate cancer xenografts: Enhanced tumor response to radiation therapy1University of Pennsylvania, Philadelphia, PA, United States, 2Thomas Jefferson University, Philadelphia, PA, United States
Synopsis
Prostate cancer, when treated with external beam radiotherapy (RT) in the range of 78 Gy, is frequently associated with gastrointestinal (GI) & genitourinary (GU) toxicities. We hypothesize that tumor sensitization by lonidamine (LND) will enable the use of lower RT doses reducing the risk of side effects. LND effects detected in vivo by 31P and 1H MRS in androgen-independent (PC3) prostate cancer xenografts produced a sustained and tumor-selective decrease in intracellular pH, bioenergetics (βNTP/Pi), oxygen consumption rate and increase in lactate. Selective tumor acidification, deenergization and oxygenation induced by LND potentiated the radiation response in the PC3 prostate cancer model.
INTRODUCTION
Early prostate cancer often presents as a multi-focal disease. Standard dose-escalated radiation therapy (RT) in the range of 78 Gy is associated with gastrointestinal (GI) & genitourinary (GU) toxicities. We hypothesize that tumor sensitization by lonidamine (LND) will enable the use of lower RT doses reducing risk of side effects in androgen-independent prostate cancer xenografts. LND inhibits the plasma membrane monocarboxylate transporters 1 & 4 (MCTs), the mitochondrial pyruvate carrier (MPC) and complex II of the electron transport chain.1, 2METHODS
PC3, hormone-independent, prostate cancer cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2mM L-glutamine, and 1% penicillin-streptomycin. 7×106 PC3 cells were inoculated subcutaneously in each mouse (n=5) as a 0.1 mL suspension.
31P and 1H MRS measurements were performed after positioning the s.c. tumor in a dual-frequency slotted-tube resonator. The intracellular pH (pHi; n=5), extracellular pH (pHe; n=5), bioenergetics (βNTP/Pi; n=5), and lactate (n=3) concentrations were measured after LND (100 mg/kg; i.p.) administration. Procedures for data acquisition, post-processing and parameter estimation were performed as previously described.3
In vitro the oxygen consumption rate and extracellular acidification rate were determined using the Seahorse XF-96 Extracellular Flux Analyzer before and after LND treatment. Glucose and lactate concentrations were measured using a YSI 2300 STAT Plus Glucose & Lactate Analyzer under the same conditions.
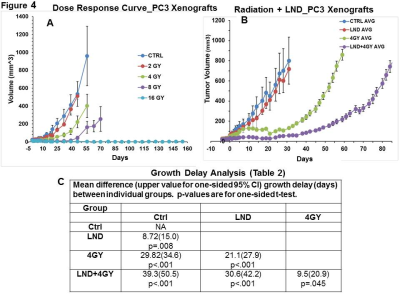
The Small Animal Radiation Research Platform (SARRP) was used to irradiate the PC3 xenografts to assess the effect of LND on RT response. Four cohorts of 5 matched animals/tumors; sham irradiated with 0, 2, 4, 8 and 16 Gy single doses were used to determine the dose-response curve measuring tumor growth delay. The 4 Gy dose was selected as providing sufficient dynamic range to demonstrate increased response with LND. The radiation dose was applied 40 min. after LND injection in order to achieve significant acidification and de-energization of the tumor. For the growth-delay determination, the tumor was monitored for 3 doubling times utilizing the log-linear regression phase of growth for each animal. Wilcoxon and t-test analysis were used to determine significant differences between treatment groups.4
The pHi, pHe, bioenergetics and lactate data at various time points following LND administration were compared by One-way ANOVA and t-test analysis.
RESULTS
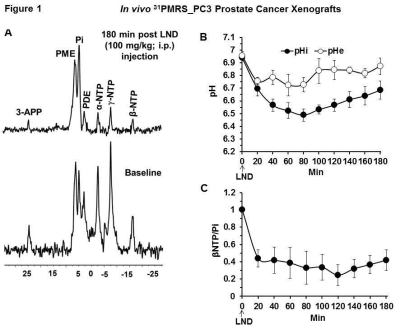
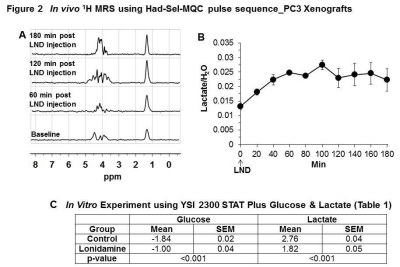
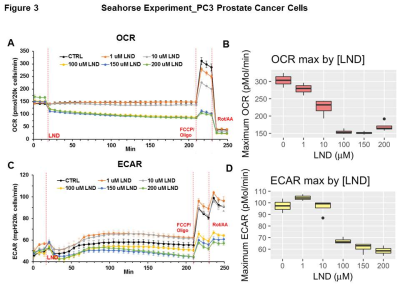
Representative localized 31P MR spectra of PC3 prostate cancer xenografts before and after LND treatment are shown in Figure 1A. Figure 1B and C show changes in pHi, pHe, and bioenergetics, respectively, in response to LND, which produced a sustained and tumor-selective decrease in pHi from 6.94 ± 0.02 to 6.49 ± 0.05 (p = 0.02), pHe from 7.06 ± 0.03 to 6.72 ± 0.08 (p = 0.08). Tumor bioenergetics (βNTP/Pi) decreased by 75.0 ± 0.12% (p = 0.01) relative to baseline immediately prior to LND administration. Figure 2A and B show representative 1H MR spectra and steady-state levels of tumor lactate, respectively (intracellular plus extracellular) monitored by 1H MRS with the Hadamard-selective multiple quantum coherence transfer pulse sequence in PC3 tumors following LND administration at time zero. Figure 2C (table 1) summarizes YSI 2300 plus glucose/lactate analyzer data on the metabolic characteristics of PC3 cells before and after LND treatment. Figure 3 shows that LND significantly inhibits respiration in PC3 cells when assayed with the Seahorse XF96 Analyzer. The addition of 100 and 150 µM LND reduced the oxygen consumption rate by 51% from 305 pMol/min to 155 pMol/min. Figure 4 demonstrates the potentiation of the radiation effect when combined with LND. For LND + 4 Gy, tumor growth delay reached 39.83 days (p<0.001) compared to 29.82 days (p<0.001) for 4 GY alone and 8.72 days (p>0.05) for LND alone (Figure 4C, table 2) demonstrating that LND had a true sensitizing effect on radiosensitivity of PC3 prostate cancer xenografts.Discussion
Acidification-induced feedback inhibition of glycolysis and intracellular acidification result in inhibition of cell growth and survival. LND also inhibits the MPC and complex-II of the respiratory chain.1, 2 In addition to causing intracellular acidification, LND causes inhibition of the oxygen consumption rate, inhibition of ATP synthesis and reduced levels of the free radical scavenger GSH. We demonstrated that these factors sensitize tumor cells to radiotherapy by a factor of 1.9.Conclusions
Tumor sensitization provided by LND may allow lowering the radiation dose by more effectively treating organ-confined disease. Therefore, our strategy, if proven effective, would avoid incomplete treatment associated morbidity of adjacent normal tissues in prostate cancer patients.Acknowledgements
McCabe Institutional Pilot Grant
References
1. Nancolas B, Guo LL, Zhou R, et al. The anti-tumour agent lonidamine is a potent inhibitor of the mitochondrial pyruvate carrier and plasma membrane monocarboxylate transporters. Biochemical Journal. 2016;473:929-36. PMCID: PMC4814305
2. Guo LL, Shestov AA, Worth AJ, et al. Inhibition of Mitochondrial Complex II by the Anticancer Agent Lonidamine. Journal of Biological Chemistry. 2016;291(1):42-57. PMCID: PMC4697178
3. Nath K, Nelson DS, Ho AM, et al. 31P and 1H MRS of DB-1 melanoma xenografts: lonidamine selectively decreases tumor intracellular pH and energy status and sensitizes tumors to melphalan. NMR Biomed. 2013;26(1):98-105. PMCID: PMC3465621.
4. Efron TB. Bootstrap Method for Standard Errors, Confidence Intervals, and Other Measures of Statistical Accuracy. Statistical Science. 1986;1(1):54-77.
Figures