3799
Longitudinal response monitoring in experimental prostate tumors by DCE-MRI after C-12 ion and photon radiotherapy1Department of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Department of Medical Physics in Radiation Oncology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 3Heidelberg Institute for Radiation Oncology (HIRO), National Center for Radiation Research in Oncology (NCRO), Heidelberg, Germany, 4Translational Radiation Oncology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 5Division of Medical Image Computing, German Cancer Research Center (DKFZ), Heidelberg, Germany, 6Department of Radiation Oncology, University Hospital Heidelberg, Heidelberg, Germany
Synopsis
Dynamic contrast-enhanced MRI was used for the longitudinal monitoring of two well characterized experimental prostate tumors (Dunning R3327-AT1 and -HI) after isodose and isoeffective radiotherapy. The effect of carbon (12C)-ion irradition compared to photons on tumor vasculature was characterized by non-compartmental analysis and pharmacokinetic modelling employing the extended Tofts model. Isodose and isoeffective irradiation experiments indicated that the beam modality has a stronger effect on tumor perfusion and permeability than the dose. While changes in perfusion were identified for the highly undifferentiated AT1-tumor, the more differentiated HI-tumor showed only minor changes in perfusion upon irradiation.
Purpose
Carbon (12C)-ion radiotherapy (RT) provides enhanced biological
effectiveness relative to photon RT, especially for the treatment of
radioresistant tumors 1,2. In this longitudinal study, we employ dynamic contrast-enhanced MRI
(DCE-MRI) to compare treatment responses of two well characterized experimental
tumors after 12C-ion and photon RT.Methods
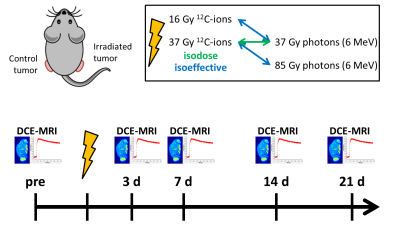
The anaplastic (AT1) and the more differentiated (HI) subline of the Dunning prostate tumor R3327 3 were transplanted subcutaneously to both thighs of male Copenhagen rats (AT1: 12 rats, HI: 16 rats). When tumors attained diameters of 10 mm, one tumor per animal was irradiated with a single dose of either photons (6 MeV) or 12C-ions (20 mm spread-out Bragg-peak), while the other served as untreated control. AT1-tumors received 37 Gy photons or 12C-ions for isodose experiments or 16 Gy 12C-ions or 85 Gy photons as isoeffective doses to 37 Gy photons and 12C-ions, respectively. The HI-tumors were irradiated with 37 Gy isodoses as well as with 18 Gy 12C-ions or 75 Gy photons (isoeffective treatments). DCE-MRI (TURBO-FLASH sequence, temporal resolution: 0.75s, 380s total acquisition) was performed one day before and 3, 7, 14, and 21 days after irradiation (Fig. 1). Image signals were converted to concentration by means of absolute signal enhancement. DCE-MRI data was analyzed voxel-wise using an in-house developed software 4 by non-compartmental analysis (normalized area-under-the-curve, AUC), mean residence time (MRT, defined as area under first moment curve) and by pharmacokinetic modelling (PKM) using the extended Tofts model (ETM) 5. The image based arterial input function was extracted individually for each animal from the left ventricle. Results were correlated with histology.Results
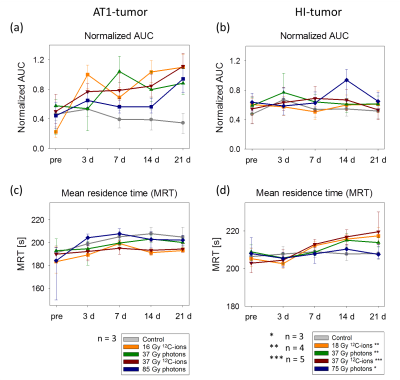
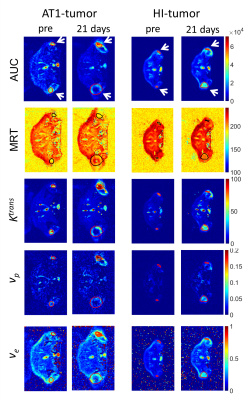
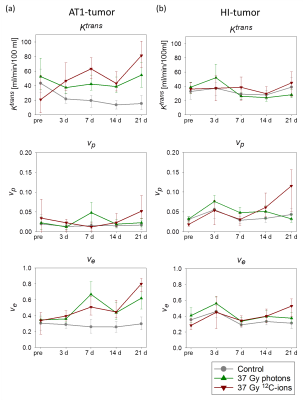
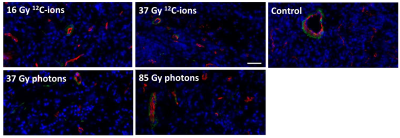
3 days after irradiation, a clear increase in AUC-values was observed for the AT1-tumors, while the control-tumors exhibited decreasing AUC-values (Fig. 2a). MRT elongated distinctly for control-AT1-tumors and slightly also for photon-irradiated AT1-tumors (Fig. 2c). Low AUC-tumor-areas corresponded with high MRT-areas (Fig. 3). 21 days after irradiation both, photon- and 12C-ion-treated groups, exhibited dose-independently similar values for AUC and MRT, respectively. For HI-tumors AUC-values remained constant while the MRT of treated tumors elongated slightly upon 7 days after treatment (Fig. 2b,d). Pharmacokinetic analysis with ETM showed a decrease in the transfer constant Ktrans for AT1-control-tumors while 12C-ion-treated tumors exhibited increasing Ktrans (Fig. 4a). Photon-treated AT1-tumors only exhibited small changes in Ktrans. HI-tumors showed less response: Ktrans-values decreased for control- and photon-treated tumors while they remained constant for 12C-ion-treated tumors (Fig. 4b). The plasma volume fraction vp, was elevated for 12C-ion-treated AT1-tumors at day 21. The interstitial volume ve, remained constant for AT1-control-tumors and showed an increase for photon and 12C-ion-treated tumors. For the HI-tumors an overall increase in vp was observed 3 days after treatment which increased further for 12C-ion-treated tumors and returned to initial values for the control and photon-treated tumors. Comparable course of parameters was observed for ve. 21 days after treatment all fit parameters exhibited maxima for 12C-ion-treated tumors. Stainings for CD31 and smooth muscle actin (SMA) revealed that irradiated AT1-tumors exhibited less but CD31+/SMA+ co-expressing vessels compared to control-tumors with a majority of small C31+/SMA- capillaries 21 days after treatment (Fig 5) 6. At the end point, HI-tumors exhibited overall larger vessels with less SMA and decreased number of vessels compared to controls.Discussion and conclusion
Low AUC- and high MRT-values in AT1-control-tumors indicate necrotic parts in the centers of the fast growing tumors induced by immature capillaries. At the same time, PKM reveals increased permeability/flow of remaining vessels in the 12C-ion-treated AT1-tumors indicated by enhanced Ktrans-values. HI-tumors showed overall fewer differences with respect to irradiation modality and dose during the 21 days observation period. Constant AUC-values and a mild elongation of MRT indicate only minor changes in tumor vasculature after treatment. For isodose experiments, 12C-ion-treated tumors exhibited larger changes in ETM-fit parameters indicating a stronger effect of 12C-ion-RT on tumor vasculature 7. For both tumor sublines, increased ve-values reflect the increasing stromal part of the tumors due to irradiation induced cell death.
Due to the AT1-tumor’s poor vascularization the ETM was chosen for pharmacokinetic analysis 8. One drawback of the ETM is the ambiguity of the parameter Ktrans which reflects both plasma flow and the capillary permeability. Due to different morphology in HI-tumors a different PK model might be more suitable (e.g. two compartment exchange model) to reveal radiation-induced changes in tissue perfusion.
DCE-MRI allows characterization of permeability changes in tumors after irradiation. Isodose and isoeffective treatments further indicate that beam modality has a stronger impact on perfusion and permeability for AT1- than for HI-tumors.
Acknowledgements
This work was supported by the German Research Foundation (DFG, KFO 214, GL 893/1-1 and KA2679/3-1). The authors are grateful to the staff of the Heidelberg Ion Therapy Center (HIT) for providing excellent working conditions.References
1. Uhl M, Herfarth K, Debus J, Comparing the use of protons and carbon ions for treatment. The Cancer Journal, 2014;20(6): 433 - 439.
2. Glowa C, Karger C, Brons S, et al., Carbon ion radiotherapy decreases the impact of tumor heterogeneity on radiation response in experimental prostate tumors. Cancer Lett, 2016;378(2): 97-103.
3. Isaacs J, Heston W, Weissman R, Coffey D, Animal model of the hormone-sensitive and -insensitive prostatic adenocarcinomas, Dunning R-3327-H, R3327-HI, and R3327-AT1. American Association for Cancer Research, 1978;38: 4353 - 4359.
4. Nolden M, Zelzer S, Seitel A, et al., The Medical Imaging Interaction Toolkit: challenges and advances. International Journal of Computer Assisted Radiology and Surgery, 2013;8(4): 607-620.
5. Sourbron S and Buckley D, Classic models for dynamic contrast-enhanced MRI. NMR Biomed, 2013;26(8): 1004-27.
6. Glowa C, In vivo Untersuchungen zur Wirksamkeit von Bestrahlungen mit Kohlenstoffionen am syngenen Prostatakarzinom Modell R3327, PhD Thesis 2013, University of Heidelberg.
7. Glowa C, Peschke P, Brons S, et al., Carbon ion radiotherapy: impact of tumor differentiation on local control in experimental prostate carcinomas. Radiation Oncology, 2017; accepted for publication DOI:10.1186/s13014-017-0914-9.
8. Sourbron S and Buckley D, On the scope and interpretation of the Tofts models for DCE-MRI. Magn Reson Med, 2011;66(3): 735-45.
Figures