3793
Fast synthetic CT generation using a conditional Generative Adversarial Network and Dixon imaging for general pelvis MR-based Radiotherapy planning1Radiotherapy Department, UMC Utrecht, Utrecht, Netherlands, 2Center for Image Sciences, UMC Utrecht, Utrecht, Netherlands, 3Image Science Institute, UMC Utrecht, Utrecht, Netherlands
Synopsis
To enable MR-only radiotherapy planning and accurate MR-based dose calculations, substitutes of CT images, so-called synthetic CT (sCT) images, need to be generated. In this work, we assessed whether sCT images generated by a 2D conditional Generative Adversarial Network (cGAN) using a 3D dual echo SPGR MR sequence were suited for radiation treatment planning for general pelvis cancer patients. Image evaluation showed comparable performance among prostate, rectum and cervix patients. Dose planning calculations demonstrated that accurate MR-based dose calculation on sCT images generated by the cGAN after training is feasible for treatment planning in prostate cancer patients. In addition, the generation of the sCT is fast (< 6 s) and ideally suited for applications where time duration is essential, e.g. MR-guided radiotherapy planning.
Purpose
To enable MR-only planning and accurate MR-based dose calculations, substitutes of CT images, so-called synthetic CT (sCT) images, need to be generated. Recently, conditional Generative Adversarial Networks (cGANs) have been proposed as a general-purpose solution to image-to-image translation problems. By interpreting the generation of sCT images as an image-to-image problem, this work aims at assessing whether a cGAN can generate sCTs from a single MR acquisition with a conventional multi-echo gradient echo sequence. Image evaluation was performed on sCT for prostate, rectum and cervix patients. In addition, a dosimetric evaluation with clinical radiation plans was performed for prostate cancer patients.Material and Methods
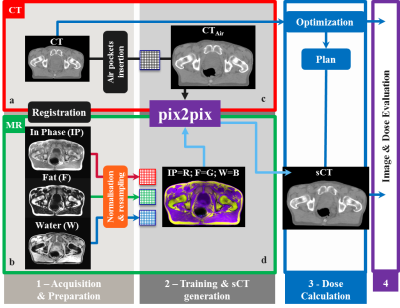
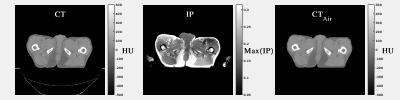
A study was conducted on 59 patients who underwent prostate external beam irradiation for which CT (Brilliance CT Big Bore, Philips Medical System, Ohio, Cleveland, USA) and MR (3T Ingenia Omega HP 5.1.7.3, Philips Healthcare, Best, Netherlands) scans were acquired on the same day and in treatment position. To generate the sCT images, dual gradient-echo, RF spoiled (SPGR), 3D T1w-MR images were reconstructed with 1x1x2.5mm3 resolution (acquired resolution was 1.7x1.7x2.5mm3), TR/TE2/TE1=3.9/2.5/1.2ms, FA=10o and BW=1078Hz/vox. Subsequently, Dixon reconstruction [1] was performed producing water (W), fat (F) and in-phase (IP) images. A cGAN called “pix2pix” [2] was used in this experiment. Before training the network, CT images were rigidly registered to MR images and MR images were normalised (Fig.1a). To keep the FOV consistent between CT and MR images, transverse slices at the top cranial and bottom caudal locations were excluded when not present in both images. Within pix2pix, all images were scaled to 8-bit grayscale values and resampled to 256x256-matrix (Fig.1b). The network was trained on 32 patients in 2D transverse slices. To guarantee consistent air pockets in the rectum and bowels on the CT and MR images during training, internal air pockets, as detected on MR images were copied to CT (Fig.1c, Fig.2) [3]. To generate sCT images, the trained cGAN was applied to the remaining 27 patients (test set). Image evaluation was performed on the sCTs in the test set using mean absolute error (MAE) as compared to CT. Dose recalculations of clinical 5-beam 10MV intensity-modulated radiotherapy plans with a prescribed dose of 35x2.0Gy to the target were performed for 15/27 patients in the test set on the CT and sCT images in Monaco (v 5.11.02, Elekta AB, Stockholm, Sweden). Dose distributions were subsequently analysed through voxel-based dose differences and gamma analysis. To verify whether the network trained on prostate patients may be used also for general pelvis, sCT generation was performed on identical Dixon images of 18 rectum and 14 cervix patients, followed by image evaluation.Results and Discussion
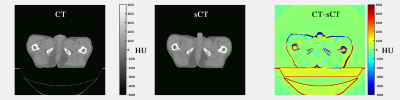
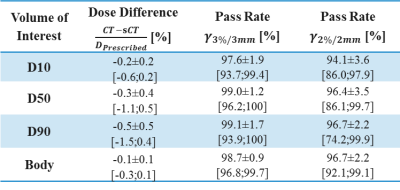
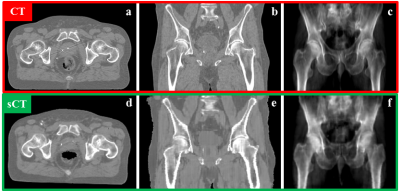
In total, 3495 slices were used for training, using 200 epochs, requiring about 11 hrs on a GPU Tesla P100 (NVIDIA, Santa Clara, CA, USA). On average each patient had a volume of about 109 slices. Applying the trained cGAN to a single patient volume required about 5.6s (Fig.3). Image Evaluation: On average, an MAE of 65±10HU (±1σ, range: 50-96HU) was obtained in the intersection of the body contours between CT and sCT. As shown in Figure3, the intrinsic differences between CT and sCT images are visible, probably due to slightly different patient set-up and different locations of internal air pockets. Dose Evaluation: On average (Tab.1), a dose difference (CT-sCT) of -0.3±0.4% (range: -1.1-0.5%) was obtained using a 50% dose threshold of the prescribed dose. A mean gamma pass rate of 96±4% was obtained. This is in line with dose differences generally accepted for MR-based radiotherapy planning [4]. Image Evaluation on general pelvis: On average, an MAE of 55±5HU (range: 38-67HU) and 59±6HU (range: 51-69HU) was obtained on 18 rectum and 14 cervix patients, respectively without retraining the cGAN. The obtained MAE is lower than for prostate patients. Possibly, the interscan difference for these patients led to an increased similarity of CT and sCT images.Conclusion
Results suggest that accurate MR-based dose calculation using a 2D cGAN for sCT generation is feasible for prostate cancer patients, and without retraining the network can also generate sCT images for rectum and cervix patients. The 2D nature of the network appears when considering the coronal plane (Fig.4): some discontinuities in the external body contour are visible between consecutive transverse slices. Future investigations will focus on extending the 2D network to 2.5 and 3D and verify the performances of the new networks. An advantage of using this network is the speed of sCT generation. Time constraints are especially relevant for MR-guided radiotherapy applications [5,6]. Given the promising results, a future study will investigate whether MR-based dose calculation on sCT obtained from the same network can be considered accurate also in the presence of magnetic fields.Acknowledgements
The research is funded by ZonMw IMDI Programme, project number: 1040030. The project is co-funded by Philips Healthcare. Max A Viergever and Jan J W Lagendijk provided general support to the research.References
[1] H. Eggers et al. 2011, Dual-echo Dixon imaging with flexible choice of echo times. Magn. Reson. Med., 65: 96-107.
[2] P. Isola et al., 2016, Image-to-Image Translation with Conditional Adversarial Networks, arXiv:1611.07004 (eprint)
[3] M. Maspero et al. 2017, Feasibility of MR-only proton dose calculations for prostate cancer radiotherapy using a commercial pseudo-CT generation method, Phys. Med. Biol. (in press).
[4] J. M. Edmund & T. Nyholm, 2017, A review of substitute CT generation for MRI-only radiation therapy. Radiat. Oncol., 12(1), 28.
[5] B.W. Raaymakers, et al., 2009, Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys.Med. Biol., 54(12):N229.
[6] S. Mutic & J.F. Dempsey, 2014, The ViewRay system: Magnetic resonance-guided and controlled radiotherapy. In Sem. Radiat. Oncol., 24(3):196-199.
Figures