3787
Metabolic alterations in DLPFC in patients with Subclinical Hypothyroidism : An In-vivo 1H MRS study1NMR Research centre, Institute of Nuclear medicine & Allied Sciences (INMAS), Delhi, India, 2Division of Thyroid Research, Institute of Nuclear medicine & Allied Sciences (INMAS), Delhi, India
Synopsis
Altered cognitive performance is well known in both hypothyroidism and hyperthyroidism but little is known about deficits in brain functions in Subclinical hypothyroidism (SCH). Aim of the present study was to investigate the metabolic changes in Dorso-Lateral Pre-Frontal Cortex (DLPFC) of SCH patients using 1H MRS. 19 freshly diagnosed SCH patients and 24 age matched healthy controls were recruited and subjected to MRS. Our result show significantly increased concentrations of GSH (p<0.009) and creatine (p<0.017) in the DLPFC and diminished cognitive performance in SCH compared to controls. The increase in the level of GSH in SCH compared to controls may be indicative of an initial compensatory, neuro-protective response due to oxidative stress.
Introduction
Subclinical hypothyroidism (SCH) is defined as mild elevation of thyroid stimulating hormone (TSH) (range 5-10 μIU/ml), normal free triiodothyronine (FT3) and free thyroxine (FT4). The brain is one of the target organs affected by thyroid hormone levels and these levels may alter the brain metabolism and function1. Diminished cognitive performance related to Attention, concentration, language, memory, psychomotor function, and executive function is well known in both hypothyroidism and hyperthyroidism2,3 but little is known about these deficits in SCH patients. Also, the necessity of hormone-replacement therapy in SCH patients is still under debate among the endocrinologists. Dorso-Lateral Pre-Frontal Cortex (DLPFC) is the seed region for memory, attention and executive functions3. MR spectroscopy (MRS) is an in vivo technique, which can identify changes at metabolic level for a very small region of interest in brain. The Aim of the present study was to investigate the metabolic changes in DLPFC due to SCH and to find correlation of these metabolic changes with cognitive performance parameters.Materials and Methods
19 freshly diagnosed SCH patients (mean age 32.5±11.26 yrs) and 24 healthy matched controls (mean age 31.84±8.93 yrs) were recruited for the study. The SCH subjects had normal FT4, FT3 levels and slightly raised TSH levels (5-10 μIU/ml). None of these patients had any history of prior trauma or psychiatric illnesses and had no contraindications for MRI examination. Spectra were acquired from dorso-lateral prefrontal cortex (DLPFC) and a voxel (12 × 12 × 12 mm3) was positioned on axial slices just above the ventricles on the right hemisphere. MRS was obtained using a point-resolved spectroscopy (PRESS) sequence with acquisition parameters: TR/TE = 2000 ms/30 ms; 2048 spectral points; 1200 Hz spectral bandwidth and 256 averages. Spectra were post-processed and concentration of the metabolites was estimated using LC model software. Data was further statistically analysed using Multivariate Analysis of Covariance (MANCOVA) using SPSS18.0, the spectra FWHM and SNR were used as covariates. The neurocognitive performance was assessed by PGI Battery for Brain Dysfunction (PGIBBD) in SCH and healthy controlsResults and Discussion
The voxel position on the axial image and representative spectra from DLPFC region in SCH are shown in figure 1A and 1B respectively. Neurocognitive assessment data using various components of PGIBBD battery in SCH patients and healthy controls are presented in Fig. 2. Although raw dysfunction score were elevated in both Nehor-Benson (NB) and Bender-Gestal (BG) test in SCH group compared to Controls (Fig 2C) but it was statistically significant only for BG test (p<0.01). Mini Mental State Examination (MMSE) score was significantly lower (p<0.01) in SCH group compared to controls (Fig 2D) and so were the other parameters related to memory (Fig. 2A) . The concentration of different metabolites visualised from DLPFC in SCH and healthy control group are shown in Fig. 3. Our result showed increase concentrations of GSH (p<0.009) and creatine (p<0.017) in the DLPFC of SCH patients as compared to controls (Fig. 3). None of the other metabolites showed any statistically significant difference in the SCH compared to control group. An earlier study has suggested that cells exposed to oxidative stress increase their antioxidant defense capacity to acclimatize and increase resistance to subsequent injury4. In another study, patients with mild cognitive impairment (MCI) had elevated GSH levels in the anterior cingulate and posterior cingulate compared to healthy subjects and the higher levels of anterior cingulate GSH was associated with poorer cognitive performance in patients with MCI5. An increase in GSH possibly will reflect either an up-regulation of local GSH production or a down-regulation in GSH breakdown to counterbalance for increased oxidative stress5. The elevated GSH level in their study is concomitant with the cognitive decline that occurs in MCI, and could reflect a neuro-protective mechanism. In our study also we have found significantly increased GSH levels (Fig.3) and increased cognitive dysfunction scores (Fig.2) in SCH patients compared to controls. The increase in GSH levels in subclinical hypothyroid (SCH) patients compared to control may suggest an initial compensatory response due to potential oxidative stress. The alterations in creatine concentration suggest abnormal bioenergetics profile in SCH subject compared to controls.
Conclusions
The present study concludes that the elevated level of GSH may be concomitant with mild cognitive decline that occurs in SCH patients and increased GSH could reflect a neuro-protective mechanism. To the best of our knowledge this is the first study reporting increased GSH levels in SCH patients. The study can be further extended to establish a marker for treatment prediction in SCH patients.Acknowledgements
The authors would like to thank Ms Prabhjyot Kaur and Mr Pawan Kumar for helping in the data acquisition. Also Mr Mukesh Kumar Saini acknowledges the fellowship grant received from DRDOReferences
1. Anderson GW. Thyroid hormone and cerebellar development. The Cerebellum. 2008; 7(1):60-74.
2. Singh S, Rana P, Kumar P, Shankar LR, Khushu S. Hippocampal Neurometabolite Changes in Hypothyroidism: An In Vivo 1H Magnetic Resonance Spectroscopy Study Before and After Thyroxine Treatment. Journal of neuroendocrinology. 2016; 28(9).
3. Zhu DF, Wang ZX, Zhang DR, Pan ZL, He S, Hu XP, Chen XC, Zhou JN. fMRI revealed neural substrate for reversible working memory dysfunction in subclinical hypothyroidism. Brain. 2006 Aug 18;129 (11):2923-30
4. Hallowell B. Role of free radicals in the neurodegenerative diseases. Drugs & aging. 2001;18(9):685-716.
5. Duffy SL, Lagopoulos J, Hickie IB, Diamond K, Graeber MB, Lewis SJ, Naismith SL. Glutathione relates to neuropsychological functioning in mild cognitive impairment. Alzheimer's & Dementia. 2014 Jan 31;10(1):67-75.
Figures
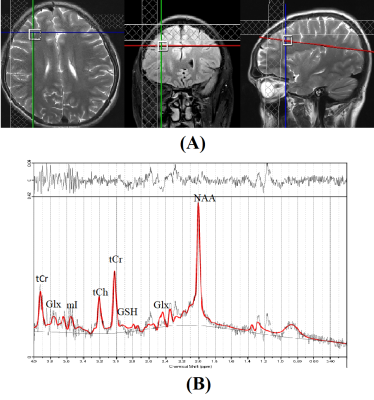
Fig.1 A) MRI image showing location of a 12mm3 voxel in right DLPFC of a SCH patient
B) Representative 1H MR spectra from right DLPFC region of a SCH patient

Fig. 2 Neuropsychological assessment of various cognitive parameters using PGIBBD showing significant decline in cognitive performance in SCH patients compared to Controls.
* indicates p<0.05 ** indicates p<0.01

Fig.3 Metabolic concentration in Right DLPFC determined by LC Model software showing significantly elevated levels of GSH and Cr in SCH patients compared to controls.
* indicates p<0.05 ** indicates p<0.01