3786
Quantitative Dynamic Susceptibility Contrasted Perfusion in Differential Diagnosis of Recurrence and Radionecrosis for Brain Metastases after Stereotactic Radiotherapy1Shandong University, Jinan, China, 2MR Collaborations NE Asia, Siemens Healthcare, Beijing, China, 3Shandong Medical Imaging Research Institute, Jinan, China, 4Shandong Provincial Hospital, Jinan, China
Synopsis
Stereotactic radiotherapy (SRS) has become a widely used treatment for patients with brain metastases (BM). However, differential radionecorsis (RN) and tumor recurrence (TR) after SRS for BM by MR images is still challenging. In this study, a quantitative dynamic susceptibility contrasted perfusion weighted imaging (DSC-PWI) technique was applied to solve this problem. The results showed that quantitative DSC-PWI had higher diagnostic efficiency and smaller inter-observer differences than traditional DSC-PWI.
Introduction
Dynamic susceptibility-contrasted perfusion-weighted imaging (DSC-PWI) is one of the most commonly used imaging methods for the diagnosis of radionecrosis (RN) and tumor recurrence (TR) after stereotactic radiotherapy [1-2]. However, traditional DSC-PWI is a qualitative or semi-quantitative method due to the sequence-acquired T2* contrast. It has higher inter-observer and diagnostic differences, which restrict intra- and inter-patient comparisons across different institutions. Bookend perfusion, which uses a pre- and post-contrast T1 map to calibrate a conventional DSC sequence, is a quantitative MR technique. It has been validated against PET and demonstrates high test-retest repeatability [3]. In this study, we applied this quantitative DSC-PWI (qPWI) method to test if the quantitative values could perform better in the differentiation of RN and TR compared with semi-quantitative values.Methods
Between March 2015 and June 2017, 46 BM patients (24 women and 22 men; median age, 61 years; age range, 30-80 years) with newly irradiated lesions after stereotactic radiotherapy (SRS) were included in this study. The patients were assigned to either the TR group or RN group based on the histopathological results (Figure 1), or the criterion Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) was applied, where a complete response, partial response, or stable disease was regarded as RN, and progressive disease was regarded as TR. All the patients underwent an MR scan on a MAGNETOM Skyra 3T scanner (Siemens Healthcare, Erlangen, Germany), using a 20-channel head-neck coil. The MR exam included a routine protocol for brain examination and a prototype quantitative DSC-PWI sequence named ScalePWI. The ScalePWI sequence merged the pre- and post-contrast T1 mapping into the GRE-EPI sequence for DSC-PWI and added the same “gradient noise” between T1 mapping and the DSC-PWI scan to avoid bias related to potential head motion. The imaging parameters of ScalePWI were as follows: TR/TE 1,600 ms/30 ms, bandwidth 1,748 Hz/pixel, 21 axial slices, field of view 220 × 220 mm2, voxel size 1.8 × 1.8 × 4 mm3, and flip angle 90 deg. For each slice, 50 measurements were acquired for the DSC-PWI analysis. After 46 seconds of injector delay, 0.2 mmol per kg body weight of contrast agent (Gd-DTPA, Magnevist; Schering, Berlin, Germany), followed by a 20-ml saline flush, were administrated. The injection velocity was 2.5 ml/s. The quantitative CBV, CBF, and MTT were inline processed immediately after the scan. The absolute cerebral blood volume of lesions (CBVlesion) and contralateral normal-appearing white matter (CBVNAWM) in both groups were measured by manually drawing ROIs. The relative CBV (rCBV) were calculated using the formula: CBVlesion/ CBVNAWM and is equal to rCBV as obtained using traditional DSC-PWI. The inter-group and intra-group differences were evaluated using the two-tailed Mann–Whitney U test and Wilcoxon-paired test, respectively. The correlation between the CBV value and rCBV was obtained using the Spearman rank correlation coefficient. The interobserver reliability was calculated using the Fleiss intraclass correlation coefficient (ICC) with a 2-way mixed model and was interpreted as follows: poor agreement, < 0.45; fair to good agreement, between 0.45 and 0.75; and excellent agreement, > 0.75. Sensitivity was defined as the ratio of accurately diagnosed recurrent metastases to the total number of recurrent metastatic lesions, and specificity was defined as the ratio of accurately diagnosed radionecroses to the total number of radionecrotic lesions. Receiver operating characteristic (ROC) curve analysis was used to determine the optimum cut-off values for the differential diagnosis of TR and RN. A p value < 0.05 was considered statistically significant.Results
The CBFlesion of TR was significantly higher than other parameters of both groups (p=0.0001, respectively, Figure 2). The ICC of CBVlesion, CBVNAWM, and rCBV were 0.932 (95%CI [0.902, 0.957]), 0.844 (95%CI [0.778, 0.916]), and 0.825 (95%CI [0.672, 0.889]), respectively. Although CBVlesion was significantly correlated with rCBV (r=0.914, p=0.001, Figure 3), a smaller correlation coefficient (r = 0.665) was demonstrated in the TR group. CBFlesion and rCBV had similar specificity (96%) in the differential diagnosis, whereas CBVlesion had a higher sensitivity (96.9% vs. 90.9%, Figure 4) and its area under the curve (AUC) was 0.973. The best cutoff value for CBVlesion was 21.8 ml/100g (specificity 96.0%, sensitivity 96.9%).Discussion and Conclusion
Quantitative DSC-PWI is a powerful method in the differential diagnosis of TR and RN of brain metastases after SRS. The absolute CBV value obtained from qPWI had a higher diagnostic efficiency and smaller inter-observer difference than rCBV as obtained from traditional DSC-PWI. Furthermore, qPWI could promote the intra- and inter-patient comparison across different institutions.Acknowledgements
No acknowledgement found.References
1. Mitsuya K, Nakasu Y, Horiguchi S, Harada H, Nishimura T, Bando E, et al. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neurooncol. 2010;99:81-8.
2. Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9:906-20.
3. Shin W, Horowitz S, Ragin A, Chen Y, Walker M, Carroll TJ. Quantitative cerebral perfusion using dynamic susceptibility contrast MRI: evaluation of reproducibility and age- and gender-dependence with fully automatic image postprocessing algorithm. Magn Reson Med. 2007;58:1232-41.
Figures
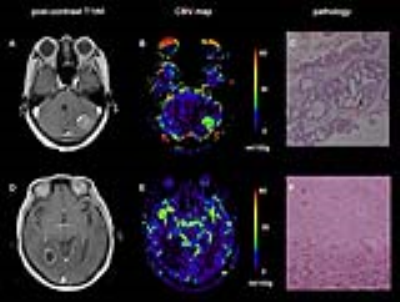
Figure 1. Illustrative cases. Patient with BM who underwent SRS five months before. A newly enhanced mass was found in an irradiated area (A); absolute CBV map showed high perfusion inside the lesion, with a mean value of 28.75 ml/100g (B); Tumor recurrence was confirmed by stereotactic biopsy. A second patient with BM had undergone SRS four months ago. An enhanced ring was revealed in the irradiated area (D); absolute CBV map showed low perfusion inside the lesion, with a mean value of 11.37 ml/100g (E); Radionecrosis was confirmed by stereotactic biopsy (F). BM, brain metastases; CBV, cerebral blood volume.

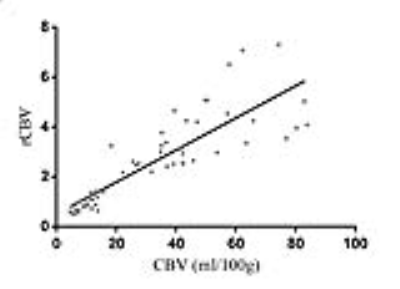
Figure 3. Spearman correlation analysis for CBVlesion and rCBV.
CBVlesion, cerebral blood volume of lesions; rCBV, relative cerebral blood volume.
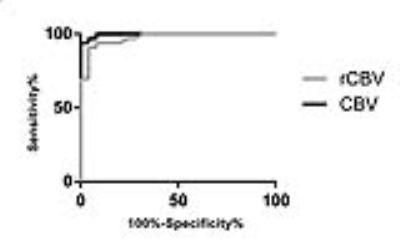
Figure 4. ROCs of CBVlesion and rCBV.
CBVlesion, cerebral blood volume of lesions; rCBV, relative cerebral blood volume.