3785
Melanopsin retinal ganglion cells in LHON patients: an fMRI study of brain activations under monochromatic light stimulationStefania Evangelisti1,2, Claudia Testa1,2, Chiara La Morgia1,3, Gilles Vandewalle4, Claudio Bianchini1,2, David Neil Manners1,2, Paola Fantazzini5,6, Michele Carbonelli3, Alfredo Sadun7,8, Caterina Tonon1,2, Valerio Carelli1,3, and Raffaele Lodi1,2
1Department of Biomedical and NeuroMotor Sciences, University of Bologna, Bologna, Italy, 2Functional MR Unit, Policlinico S.Orsola - Malpighi, Bologna, Italy, 3IRCCS Institute of Neurological Sciences of Bologna, Bologna, Italy, 4Sleep Research Group; GIGA-Research, Cyclotron Research Centre/In vivo imaging unit, University of Liège, Liège, Belgium, 5Department of Physics and Astronomy, University of Bologna, Bologna, Italy, 6Centro Enrico Fermi, Roma, Italy, 7Doheny Eye Institute, University of California, Los Angeles, CA, United States, 8Doheny Eye Centers of UCLA, Department of Ophthalmology, David Geffen School of Medicine at University of California, Los Angeles, CA, United States
Synopsis
We combined light stimulation and fMRI to investigate the contribution of melanopsin-expressing retinal ganglion cells (mRGCs) to visual and non-visual processes in Leber Hereditary Optic Neuropathy (LHON) paradigm of retinal degeneration . Monochromatic visual simulation showed a stronger effect in LHON visual cortex for blue vs red light with relatively long stimuli, supporting the hypothesis of a role for melanopsin in visual processes. When light was combined with a working memory task, blue light modulation of cognitive brain response was maintained in LHON; indeed the effect was stronger than in healthy subjects, probably because of the higher mRGCs/RGCs ratio in LHON retinas.
Introduction
A third photoreceptor type, different from rods and cones, exists in mammalian retina: melanopsin-expressing retinal ganglion cells (mRGCs). Melanopsin plays a key role in non-image-forming functions and is maximally sensitive to blue light1,2. MRGCs are thought to play a prominent role in mediating fMRI attentional brain response during blue light stimulation3,4 but definite proof is difficult to obtain in healthy volunteers. Leber Hereditary Optic Neuropathy (LHON) is a mitochondrial genetic disease that manifests with loss of central vision due to RGC degeneration and consequent optic nerve atrophy5,6. Despite the loss of RGCs, those expressing melanopsin are relatively spared7 such that patient show a relative increase in mRGC photoreception compared to classical visual photoreception. The aim of this study was to assess mRGC contribution to visual and non-visual processes in LHON paradigm of retinal degeneration by studying cerebral fMRI activation patterns during light stimulation.Methods
We evaluated 13 patients with genetically confirmed diagnosis of LHON and 13 matched healthy controls (HC, Tab.1). All participants underwent a brain MR-protocol (1.5T GE scanner) including visual and visual-cognitive fMRI paradigms (TR=3s, resolution=1.875x1.875x4mm) and volumetric T1-w acquisition (1mm3). The pure visual task consisted of a pseudorandom alternation of blue, red and dark periods (Fig.1). The visual-cognitive task was an independent combination of light stimulation and auditory 0/3-back task (Fig.2). Blue (480nm) and red (620nm) lights were transmitted using a specific instrumental setup6. Light intensities were set to produce the same photon flux at the eye (5x1013ph cm−2s−1) at both wavelengths. Paradigms were presented using COGENT-2000 (MATLAB). Before acquisitions, participants were asked to follow a regular sleep-wake rhythm for one week. Pupils were chemically dilated before participants were blindfolded in the dark for 1 hour before fMRI recordings. Analyses were performed with FSL. Pre-processing included motion correction, high-pass filtering (100s), spatial smoothing (FWHM=5mm) and slice-timing correction. A general linear model was applied, modelling the effects of interest with box-car functions convolved with a double-gamma HRF. Stick functions were added to model light onsets/offsets. For the visual-cognitive paradigm, interactions between light and task were investigated. Linear modulated regressors were also added to the design. Functional images were registered to T1-w images (BBR) and T1-w images were non-linearly (FNIRT) aligned to MNI. Group comparisons were performed with FLAME-1 mixed-effect analysis, with age, sex and contemporaneous daylight length as nuisance regressors. Statistical maps were corrected for multiple comparisons (cluster z=2.3, FWE p<0.05).Results
Visual stimulation results were considered for different durations of light stimuli: transient effects, 10s and 50s sustained effects (Fig.3). At light onset, the visual cortex was activated for both groups and both colors. Regarding occipital cortex modulation, with 10s sustained stimuli a significant LHON group activation was lacking for red, while no group differences were present with blue. With longer sustained effects (50s), visual cortex activations were present in both groups with both colors, but without group differences for blue, while a lower activation was found for red in LHON. The most straightforward result was that brain response was higher with blue vs red in the occipital pole for LHON. Regarding the cognitive paradigm, all the participants got at least 75% of correct answers, without group or light effects; brain activations, irrespectively of light conditions, were in line with fMRI literature for n-back8 and analogous between groups (data not shown). As for the interactions between light and task, we found no light modulation effect on HC cognitive response, while a higher brain activity was found in LHON when the task was performed under blue light compared to red, mainly in middle and inferior frontal gyri, temporo-occipital regions, insula, putamen, cerebellum, and, with lower significance, brainstem (Fig.4).Discussion and conclusions
With purely visual simulation, a stronger effect in visual cortex for LHON with blue vs red became clearer as the duration of the stimuli got longer. Considering the sluggish and sustained response of melanopsin, these support the hypothesis of a role for melanopsin in visual processes9. The blue light modulation of cognitive brain response we observed in LHON was in brain regions that were consistent with previous results3,4. We saw a stronger effect in LHON probably due to the higher ratio of mRGCs to RGCs in their retinas7. These results showed that mRGCs’ function of modulating brain activity during a working memory task was maintained in LHON. In conclusion, this first fMRI study in LHON patients demonstrates that mRGCs maintained functionality, and also provides a good paradigm of retinal degeneration to study human mRGCs in-vivo.Acknowledgements
No acknowledgement found.References
- Provencio I, Rodriguez IR, Jiang G et al. A novel human opsin in the inner retina, Journal of Neurosciences 2000; 20: 600-605.
- Hankins MW, Peirson SN, Foster RG. Melanopsin: an exciting photopigment, review, Trends in neurosciences 2007; 31 (1): 27-36.
- Vandewalle G, Schmidt C, Albouy G et al. Brain responses to violet, blue and green monochromatic light exposures in humans: prominent role of blue light and the brainstem, Plos One 2007; 2(11): e1247.
- Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function, Trends in cognitive sciences 2009; 13(10):429-438.
- Man PYW, Turnbull DM, Chinnery PF. Leber hereditary optic neuropathy. J Med Genet, 39(3):162–9, 2002
- Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res, 23(1):53–89, 2004.
- La Morgia C, Ross-Cisneros FN, Sadun AA et al. Melanopsin retinal ganglion cells are resistant to neurodegeneration in mitochondrial optic neuropathies, Brain 2010; 133: 2426-2438
- Owen AM, McMillan KM, Laird AR et al. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp, 25(1):46–59, 2005
- Brown TM, Gias C, Hatori M et al. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol, 8:e1000558, 2010
Figures
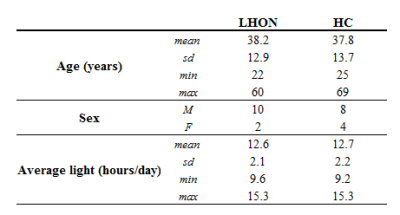
Table 1. Demographic summary of patients and healthy
controls. Average light refers to the average number of hours of light per-day
at the time of MR session (data from https://weatherspark.com/averages, Bologna
Airport Guglielmo Marconi Airport weather station).

Figure 1. Pure visual paradigm: purely visual stimulation with
a pseudorandom alternation of monochromatic blue and red light and dark periods.
Light and dark periods last, respectively, 10 and 5 seconds.
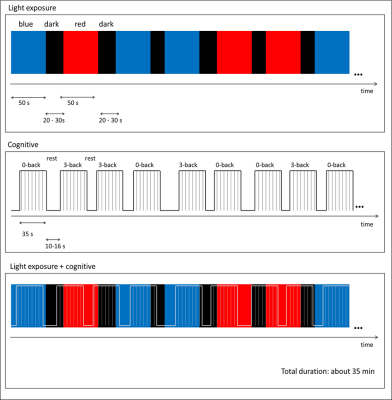
Figure 2. Visual Cognitive paradigm: monochromatic light
stimulation (top panel) is combined with an alternation of 0-back and 3-back
task (middle panel). Light periods lasted 50s, while darkness was randomly
between 20 and 30s. Each auditory task block consisted of a series of 14
stimuli, lasting 35s, and was alternated to rest periods lasting 10 to 16s. Stimuli
consisted of nine Italian monosyllabic consonants. The cognitive part of the paradigm was
simultaneous, but totally uncorrelated, to the light stimulation (bottom panel).
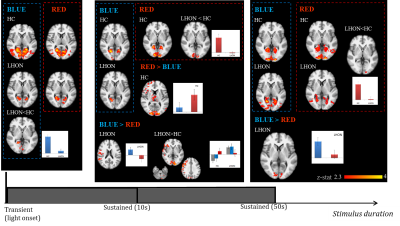
Figure 3. Group-level results for the purely visual
stimulation: transients effects at light onset (left panel), 10s sustained
effects from the pure visual paradigm (middle panel) and 50s sustained effects
from the visual-cognitive paradigm (right panel). Statistical maps of z-values
are shown (FWE corrected, p<0.05). The background image is an average of
individual T1-w scans, images are shown in radiological convention. Histograms of
the estimated response for representative clusters are reported.
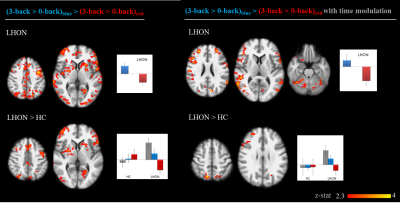
Figure 4. Group-level results for the interaction
between light conditions and attentive task for the visual-cognitive fMRI paradigm.
Statistical maps of z-values are shown (FWE corrected, p<0.05). The
background image is an average of individual T1-w scans, images are shown in
radiological convention. Histograms of the estimated response for representative
clusters are reported.