3783
White matter microstructural maturation in adolescent American Football athletes is affected by history of sport-participation and concussion1Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 2College of Veterinary Medicine, Purdue University, West Lafayette, IN, United States, 3Electrical and Computer Engineering, Purdue University, West Lafayette, IN, United States, 4Department of Basic Medical Sciences, Purdue University, West Lafayette, IN, United States, 5Department of Health and Kinesiology, Purdue University, West Lafayette, IN, United States, 6Department of Mechanical Engineering, Purdue University, West Lafayette, IN, United States
Synopsis
Concussion is a biomechanically induced brain injury that causes mental health concern for adolescent collision-sport athletes, whose brains are still developing and maturing. These athletes normally continue participating in collision events, regardless of history of concussion and risk for future concussion. Using diffusion tensor imaging, we investigated 93 asymptomatic adolescent athletes who participate in high school American Football. Per developmental expectations, we typically observed increasing fractional anisotropy or decreasing mean diffusivity with increasing years of high school experience, but prior history of concussion reversed this trend in a number of brain regions.
Introduction
Concussion is a biomechanically induced brain injury that affects sports athletes,1 especially adolescents.2 Adolescents undergo a critical period of brain development,3,4,5 white matter (WM) continues to mature,6,7,8 and continued participation in contact sport activities raises concern for their mental health. Recent cross-sectional diffusion tensor imaging (DTI) studies reported decreased mean diffusivity (MD) and/or increased fractional anisotropy (FA) in several WM fiber tracts in adolescent9,10 and adult athletes11 with a history of concussion. This DTI study investigates if and how history of participation (years of high school experience) and concussion correlate with WM microstructural maturation in adolescent athletes.Methods
Ninety-three male, high school varsity football athletes (ages: 14-18; mean=16.1) participated in the 2015 and 2016 seasons of the long-term study by the Purdue Neurotrauma Group. At the time of enrollment, 13 participants reported no high school football experience (0Y), 19 reported one year (1Y), 28 reported two years (2Y), and 33 reported three years of play (3Y). A history of diagnosed concussion (HOC+) was reported by 28 participants. All subjects underwent an MRI session prior to the beginning of contact practices, in which diffusion-weighted images were acquired with a 3 Tesla General Electric Signa HDx and 16-channel brain array (Nova Medical), using a spin-echo, echo-planar imaging sequence (TE=100ms, TR=12,500ms, 40 slices, 2.5mm thickness, matrix=96×96, upsampled to a resolution of 1mm isotropic) with 30 diffusion-encoding directions at b=1000s/mm2 and one volume at b=0s/mm2. Data were preprocessed using FSL (FMRIB 5.0), including motion and eddy current corrections, followed by brain extraction. Fractional anisotropy (FA) and mean diffusivity (MD, units: mm2/s) were estimated for each individual, and the data underwent visual quality inspection. Tract-based spatial statistics (TBSS)12 were used to create mean FA and MD skeletons. Voxel-wise statistics with 5000 permutations were performed in a pairwise manner among the 0Y, 1Y, 2Y, and 3Y groups, with threshold-free cluster enhancement and family-wise error correction for multiple comparisons (p<0.05). For multivariate linear regression analyses, mean FA and MD skeletons from TBSS were segmented into 48 ROIs defined in the JHU-ICBM-DTI-81 WM label atlas.13 Segments with no FA or MD values, and segments rejected by the Shapiro-Wilks normality test were excluded. Independent variables were years of experience, history of concussion, and age. False discovery rate correction was used to control the type-I error for multiple comparisons (adjusted p threshold = 0.026).Results
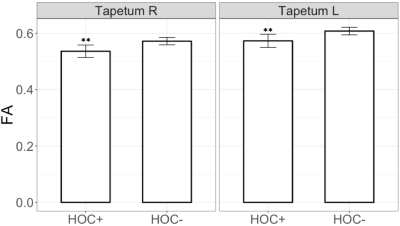
Voxel-wise statistics computed across experience groups exhibited widespread clusters within temporal-parietal and frontal WM fibers, showing contrasts where FA was higher (Figure 1A and 1B) and MD was lower (Figure 1C, 1D, and 1E) for groups with more years of experience. Multivariate linear regression analyses showed a significant interaction (F[3,106]=4.34, p=0.006) between years of high school experience and history of concussion in the left stria terminalis, where HOC+ exhibited lower FA as years of high school experience increased (Figure 2). Similar to the findings of voxel-wise statistics, regression analyses in football athletes showed increasing FA or decreasing MD with more years of experience (Table 1). HOC+ exhibited decreasing FA in the bilateral tapetum with increasing years of experience (both β<-0.03; p<0.009, see Figure 3), which contrasted with the finding in non-concussed football athletes.Discussion
Both the voxel-wise statistics and multivariate linear regression analyses showed an increase of FA and decrease of MD as a function of years of high school experience within multiple brain regions (Figure 1 and Table 1), which is consistent with WM maturation during adolescence.6,7,8 In contrast, HOC+ football athletes exhibited WM microstructural deficits within the fiber tracts belonging to posterior temporal lobes and limbic pathway (Figures 2 and 3), suggesting demyelination or other membrane damage may have occurred within those regions,14 suggesting that the WM maturation process can be perturbed by injury. Such damage could lead to dysfunction in controlling stress and emotional behavior15 which has long been believed to exacerbate the injury risk in adolescent athletes.16 While the HOC+ findings contrast with much of the previous literature,9,10,11 most of these studies suffer from heterogeneity in participant sport, age, number of concussions, time since last concussion, and exposure to subconcussive events.Conclusion
Using a large cohort of asymptomatic football athletes, it has been observed that the WM maturation processes during adolescence may be affected by an individual’s history of participation and concussion. As these athletes participate in successive years, their brains may develop and mature per usual, but the experience of a diagnosed concussion can adversely affect brain structure, raising concerns for long-term mental health.Acknowledgements
This work was supported by grant from the General Electric Healthcare (PIs: Drs. Thomas M. Talavage, Eric A. Nauman, Larry J. Leverenz), and by the use of resources and facilities at InnerVision Imaging Center. We thank Dr. Gregory G. Tamer, Jr., for the training and assistance in scanner operation and data collection.References
1. Maroon J, Lepere D, Blaylock R, et al. Postconcussion syndrome: a review of pathophysiology and potential nonpharmacological approaches to treatment. Phys Sport. 2012; 40:73–87.
2. Orchard J, Wood T, Seward H, Broad A. Comparison of injuries in elite senior and junior Australian football. J Sci Med Sport. 1998; 1:83–88.
3. Asato M, Terwilliger R, Woo J, et al. White matter development in adolescence: a DTI study. Cereb Cortex. 2010; 20(9): 2122-2131.
4. Ladouceur C, Peper J, Crone E, et al. White matter development in adolescence: The influence of puberty and implications for affective disorders. Developmental Cognitive Neuroscience. 2012; 2(1): 36-54.
5. Pfefferbaum A, Mathalon D, Sullivan E, et al. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994; 51(9): 874-887.
6. Barnea-Goraly N, Menon V, Eckert M, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005; 15(12): 1848-1854.
7. Lebel C, Beaulieu C. Longitudinal Development of Human Brain Wiring Continues from Childhood into Adulthood. Journal of Neuroscience. 2011; 31(30): 10937-10947.
8. Schmithorst V, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain and Cognition. 2010; 72(1): 16-25.
9. Manning K, Schranz A, Bartha R, et al. Multiparametric MRI changes persist beyond recovery in concussed adolescent hockey players. Neurology. 2017; [Epub ahead of print].
10. Jang I, Zou Y, Nauman E, et al. Diffusion Tensor Imaging Reveals Persistent Effects on White Matter Microstructure in High School Football Players with History of Sports-Related Concussion. Proc. Int. Soc. Magn. Reson. Med. 2012.
11. Churchill N, Hutchison M, Richards D, et al. Brain Structure and Function Associated with a History of Sport Concussion: A Multi-Modal Magnetic Resonance Imaging Study. J Neurotrauma. 2017; 34(4): 765–771.
12. Smith S, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006; 31:1487-1505.
13. Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2016; 51(5): 527-539.
14. Jones D, Knösche T, Turner R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. NeuroImage. 2013; 73:239-254.
15. Gungor N, Paré D. Functional Heterogeneity in the Bed Nucleus of the Stria Terminalis. J Neurosci. 2016; 36(31): 8038–49.
16. Williams J, Andersen M. Psychosocial antecedents of sport injury: Review and critique of the stress and injury model. J Applied Sport Psychology. 1998; 10(1): 5-25.
Figures
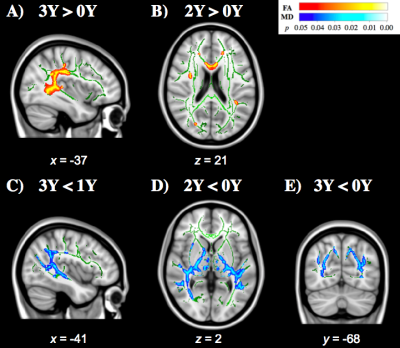
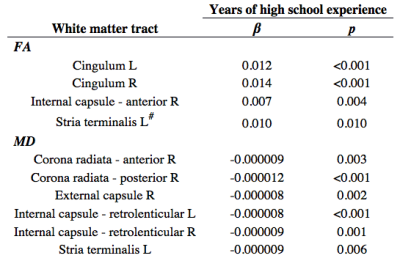
Table 1. Multivariate linear regressions for regional fractional anisotropy (FA) and mean diffusivity (MD) of adolescent athletes with 1-3 years of high school experience (1Y, 2Y, 3Y). R/L: right/left hemisphere. #: significant interaction with history of concussion.
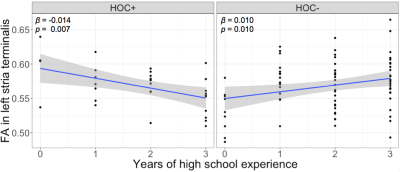
Figure 2. Line graphs illustrating the significant interaction between years of high school experience and history of concussion (HOC+: with history of concussion; HOC-: without history of concussion) on fractional anisotropy (FA) in the left stria terminalis. The corresponding slopes (β) and p values are labeled at the top-left corners.