3779
Altered Hippocampal Structure and Functional Connectivity Associated with Memory Impairment in Patients with End-stage Renal DiseaseXueying Ma1, Dun Ding1, Peng Li2, Pan Zhang1, and Ming Zhang1
1Department of Medical Imaging, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China, 2Department of Medical Imaging, NO.215 Hospital of Shaanxi Nuclear Industry, Xianyang, China
Synopsis
Memory impairment was common among ESRD patients, but the physiopathologic mechanisms were largely unclear. Hippocampus and prefrontal cortex played an important role in the human memory. Here we analyzed the structurre and functional connectivity of hippocampus and its correlation with memory scores (AVLT-H). We found that the hippocampal volume and FC between the rostral/caudal hippocampi and ventrolateral prefrontal cortex, dorsolateral prefrontal cortex as well as middle temporal gyrus. Correlations were found between hippocampal changes and memory impairment. Our study indicated that the hippocampus should be taken into consideration in the further mechanism study of neurol damage in ESRD patients.
Introduction
End-stage renal disease (ESRD), as the last stage of chronic kidney disease (CKD), is characterized by an estimated glomerular filtration rate (eGFR) less than 15 ml/min/1.73 m2 and the need for renal replacement therapy via dialysis and transplantation.1 The impaired memory was reported affected up to 30% ESRD patients and could complicate ESRD treatment.2,3 However, the underlying neuropathological mechanisms of impaired memory in ESRD patients were largely unclear. Accumulating evidence has demonstrated hippocampus and prefrontal cortex (PFC) played an important role in the human memory from the moment of encoding to retrieval.4 Neuroimaging studies have found hippocampal volume was decreased in ESRD patients which was associated with cognitive impairment.5 In this study, we used three-dimensional T1-weighted and resting functional MR imaging (resting fMRI) to to detect the structural and functional changes of hippocampus, focusing on exploring the relationships between memory impairement and hippocampal changes in ESRD patients. Pathophysiological factors intrinsic to the uremic state and diaysis procedure might be contributing to the memory impairment in ESRD patients. In order to exclude the confounding factors in diaysis procedure, we included ESRD patients without dialysis.Methods
Thirty-five ESRD patients without diaysis (23 men, 12 women; aged 20-48 years, mean age 32.79±9.29 years) and thirty-five age-/gender-matched healthy volunteers (19 men, 16 women; aged 20-49years, mean age 34.33±9.71 years) were included in our study (Table). All of the subjects underwent the Auditory Verbal Learning Test-Huashan version (AVLT-H) to evaluate the episodic memory conditions, which included learning, short-term recall (SR), long-term recall (LR) memory and recognition. Three-dimensional T1-weighted imaging and resting fMRI were acquired using a GE 3.0T HDxt scanner. Hippocanpal volume was calculated used FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/). Seed-based functional connectivity (FC) analysis was performed used Data Processing Assistant for Resting-State fMRI toolbox (http://www.restfmri.net/forum/DPARSF) and statistical parametric mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm) was conducted for the statistical analysis.6 For statistical analysis, memory scores were compared between patients and controls using an unpaired Student’s t-test. Correlations between hippocampal volume and memory scores were conducted using partial correlation analysis.Results
ESRD patients displayed significant decreased volume of hippocampi campared with control subjects (left hippocampus, 3895.59±446.97 versus 4131.72±345.57, P<0.05; right hippocampus, 3921.77±366.37 versus 4405.26±405.65, P<0.01) (Figure1). The volume of hippocmpus was correlated with AVLT scores ( P<0.05). Based on the freesurfer region of interest (ROI) analysis results, we regarded bilateral rostral and caudal hippocampus (rHipp and cHipp) as the seeds respectively7. ESRD patients showed decreased FC between the bilateral rHipp and bilateral ventrolateral prefrontal cortex (VLPFC) and left middle temporal gyrus (MTG) (Figure2). Decreased FC was found between left cHipp and right VLPFC, and also observed between right cHipp and right VLPFC and dorsolateral prefrontal cortex (DLPFC) (Figure2). For the interrelationship between the severity of memory impairment and the FC changes of bilateral rHipp to whole brain, we found that FC between bilateral rHipp and medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC) was positively correlated with SR and LR scores (Figure3). No significant correlation was found between FC of bilateral cHipp to PFC and memory scores (Figure3).Discussion
The hippocampus is considered as the hub of brain network communication for memory, standing out as a crucial structure for the initial encoding and storage of labile memories.8 Neurol electrophysiological and neuroimaging studies showed that regions of PFC and MTG involved during memory maintenance and retrieval, especially for the regions of VLPFC, DLPFC and mPFC.8 Animal and human studies have revealed that PFC associated to the hippocampus by receiving monosynaptic excitatory and plastic input connections, which was was important during memory-related activities.8 Our results indicated that the memory impairment in ESRD patients involved the connections changes of hippocampus to PFC. The underlying pathophysiological connection between renal impairment and brain function might contribute to the FC and structural changes in ESRD patients.9Conclusion
The altered hippocampal structure and FC and its association with memory impairment were detected in ESRD patients, indicating that the hippocampus should be taken into consideration in the further mechanism study of neurol damage in ESRD patients.Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. 81371530 and 81571640) and Natural Science Foundation of Shaanxi Province of China (Grant No. 2017ZDJC-13).References
- Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol 2007;18(10):2644-8.
- Dong J, Pi HC, Xiong ZY, Liao JL, Hao L, Liu GL, et al. Depression and Cognitive Impairment in Peritoneal Dialysis: A Multicenter Cross-sectional Study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2016;67(1):111-8.
- McQuillan R, Jassal SV. Neuropsychiatric complications of chronic kidney disease. Nat Rev Nephrol 2010;6(8):471–479.
- Battaglia FP, Benchenane K, Sirota A, Pennartz CM, Wiener SI. The hippocampus: hub of brain network communication for memory. Trends in cognitive sciences. 2011;15(7):310-8.
- Chang CY, Lin CC, Tsai CF, Yang WC, Wang SJ, Lin FH, et al. Cognitive impairment and hippocampal atrophy in chronic kidney disease. Acta neurologica Scandinavica. 2017;136(5):477-85.
- Chao-Gan, Y. & Yu-Feng, Z. (2010). DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front Syst Neurosci, 413.
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cerebral cortex (New York, NY : 1991). 2016;26(8):3508-26.
- Battaglia FP, Benchenane K, Sirota A, Pennartz CM, Wiener SI. The hippocampus: hub of brain network communication for memory. Trends in cognitive sciences. 2011;15(7):310-8.
- Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. Journal of the American Society of Nephrology: JASN. 2013;24(3):353-63.
Figures
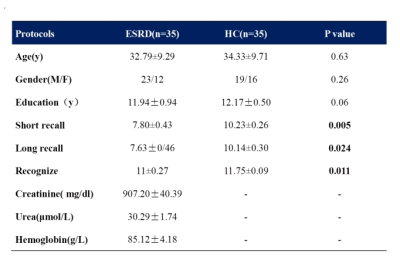
Table Demographic and clinical characteristics of ESRD patients and healthy controls
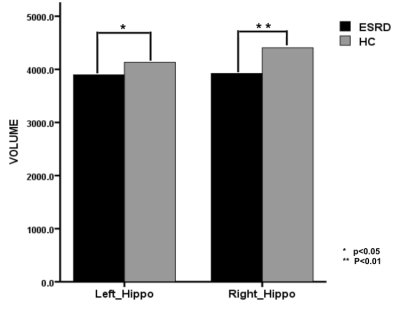
Figure1 Decreased hippocampal volume in ESRD patients compared with healthy controls.
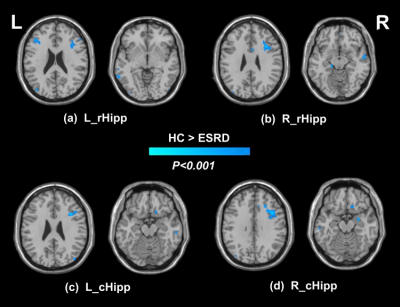
Figure2 Decreased functional connectivity of ESRD patients in contrast to controls between the rostral/caudal hippocampal seed and DLPFC, VLPFC and MTG.
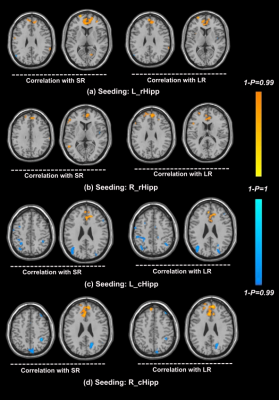
Figure3 Correlations between functional connectivity of the rostral/caudal hippocampal seed and SR and LR memory in ESRD patients. Yellow color indicates that the SR and LR memory were positively correlated with functional connectivity of the rostral/caudal hippocampi in mPFC and ACC. Blue color indicates that the SR and LR memory were negatively correlated with functional connectivity of the caudal hippocampi in bilatiral inferior parietal lobule.