3773
Predictive value of QSM for hippocampal atrophy in pre-clinical Alzheimer’s disease1CSIRO Health and Biosecurity, Australian E-Health Research Centre, Brisbane, Australia, Brisbane, Australia, 2Cooperative Research Centre for Mental Health, Parkville, Australia, Melbourne, Australia, 3Melbourne Dementia Research Centre, Florey Institute of Neuroscience and Mental Health, The University of Melbourne, Parkville, Australia, Melbourne, Australia, 4Austin Health, Heidelberg, Australia, Melbourne, Australia, 5Department of Medicine and Radiology, Royal Melbourne Hospital, University of Melbourne, Parkville, Australia, Melbourne, Australia, 6Academic Unit for Psychiatry of Old Age, University of Melbourne, Melbourne, Australia, Melbourne, Australia, 7National Ageing Research Institute, Melbourne, Australia, 8Cogstate, Melbourne, Australia, Melbourne, Australia, 9Centre of Excellence for Alzheimer’s Disease Research and Care, Edith Cowan University, Joondalup, Australia, Melbourne, Australia, 10Department of Anatomy and Neuroscience, The University of Melbourne, Parkville, Australia, Melbourne, Australia, 11https://aibl.csiro.au, Melbourne, Australia
Synopsis
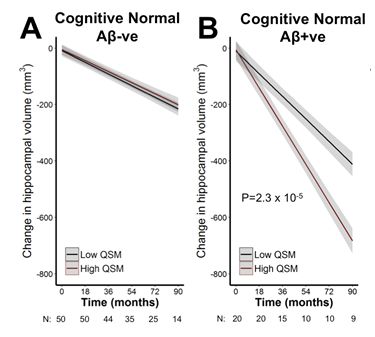
One-third of cognitively normal people over the age of 65 exhibit β-amyloid plaques, a defining pathology of Alzheimer’s disease. The hippocampus also undergoes early and pronounced neurodegeneration in Alzheimer’s disease, which underlies the memory impairment. Cognitively normal people with high β-amyloid pathology are at risk of hippocampal neurodegeneration, but the rate of decline is variable between subjects. Here, we investigate whether the iron load of the hippocampus can be used to stratify risk for future hippocampal atrophy in cognitively normal people with and without β-amyloid. We applied Quantitative Susceptibility Mapping (QSM), a relatively new MRI modality that is sensitive to tissue iron levels, to 70 cognitively normal people who also had a PET scan for β-amyloid, and were monitored for brain volume changes in MRI scans performed every 1.5 years for up to 7.5 years. We found that QSM of the hippocampus was strongly predictive of future atrophy of this region in cognitively normal subjects who had high β-amyloid pathology (P=2.3x10-6), but not in cognitively normal subjects with low pathology. These data support a role for iron in contributing to neurodegeneration in Alzheimer’s disease, and QSM in combination of β-amyloid PET scans could be used to stratify patients at risk for cognitive decline in the pre-symptomatic phase.
Introduction
High ß-Amyloid (Aβ) burden, a defining pathology of Alzheimer’s disease, is observable using PET imaging in ~30% of people over the age of 65 who are cognitively normal. In this pre-clinical stage, the Aβ load is associated with an increased rate of hippocampal atrophy, but the rate of neurodegeneration is highly variable between subjects 1, which might suggest that other pathological changes in Alzheimer’s, such as iron, might combine with Aβ to promote neurodegeneration. Non-invasive iron imaging technique through quantitative susceptibility mapping (QSM) MRI has already shown promising results in predicting cognitive decline in people with Aβ pathology 2. In this study, we investigated whether the iron load in the hippocampus and other cortical brain regions could be used to stratify the risk for future atrophy in cognitively normal people with high and low Aβ pathology.Method
Dataset: For this study, 70 cognitively normal participants from the Australian Imaging, Biomarkers & Lifestyle (AIBL) cohort were selected. These subjects received 11C-PiB-PET, T2*- and T1W-MRI imaging at baseline, and also T1W every 18 months for up to 7.5 years.
Image Analysis: The Aß status was determined using CapAIBL© 3, considering the grey-matter cerebellum uptake as the reference to obtain a standardized uptake value (SUVR). The neocortical retention cut-off of 1.5 was used to group subjects into low and high Aß. The 3D T1W MPRAGE data was processed to generate anatomical brain parcellation maps using an atlas-based approach. The 3D T2*W single echo GRE (TE/TR=20/27 msec) acquisition with available phase and magnitude images for each head coil channel was used for QSM post-processing. First, a brain mask was generated from the bias-field corrected magnitude data using FSL-BET. A Laplacian-based method was used to unwrap each coil phase image followed by background field elimination using vSHARP approach 4. The corrected phase images were then combined by weighting the magnitude of the corresponding channel. STI Suite software (v2.2) was used for QSM reconstruction 5. The regional volume and QSM values were normalized using total intracranial volume (GM+WM+CSF) and middle-frontal white matter as suggested in 6, respectively.
Statistical Analysis: For statistical analysis, mixed-effects linear models were used to assess the relationship between baseline QSM and longitudinal volume changes in several brain regions. The patient groups were stratified by Aβ status and were adjusted for: age, sex, APOE ε4, and neocortical SUVR (as a continuous variable).
Results
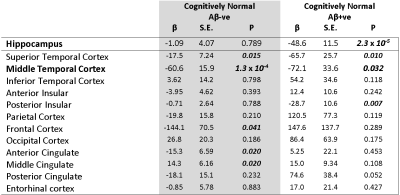
We found that hippocampal QSM was associated with accelerated hippocampal atrophy in cognitively normal subjects with Aβ pathology (β[S.E.] = -48.6 [11.5]; P= 2.3 x 10-6). For display purpose, the data-points representing volume-QSM were stratified according to median hippocampal QSM values (Figure 1). Baseline hippocampal QSM was not associated with hippocampal atrophy in cognitively normal subjects without Aβ pathology (P= 0.789). In exploratory modelling of other brain regions, QSM was also predictive of future atrophy in, for example, the temporal and insular lobes (Table 1).Discussion
The hippocampus exhibits early and pronounced neurodegeneration in
Alzheimer’s disease, but the rates of atrophy vary between individuals. By
combining QSM-MR imaging with β-amyloid-PET imaging we found that high
hippocampal iron predicted longitudinal atrophy in cognitively normal subjects
with high Aβ load. In contrast, higher values of hippocampal QSM without Aβ
were not associated with neurodegeneration. Iron, therefore, appears to become associated
with toxicity in the hippocampus when Aβ is present, possibly through increased
oxidative stress.
While QSM was considered as an imaging marker that is sensitive to iron, it
may not be a direct measure of brain iron, as QSM signal can also be affected
by other factors such as cortical composition, myelin, calcification, head orientation
and scanner calibration. However, our QSM results are consistent with previously reported trends based on CSF
ferritin 7. Our findings suggest that QSM could be used alongside
β-amyloid PET imaging as a stratification tool to identify suitable
pre-clinical patients for clinical trials,
or could be used in clinical settings.Acknowledgements
The AIBL study thanks the participants and the clinicians who referred them. The AIBL study (www.AIBL.csiro.au) is a consortium between Austin Health, CSIRO, Edith Cowan University, the Florey Institute (The University of Melbourne), and the National Ageing Research Institute. Partial financial support was provided by the Alzheimer’s Association (US), the Alzheimer’s Drug Discovery Foundation, an anonymous foundation, the Science and Industry Endowment Fund, the Cooperative Research Centre for Mental Health, the Dementia Collaborative Research Centres, the Victorian Government Operational Infrastructure Support program, the McCusker Alzheimer’s Research Foundation, the National Health and Medical Research Council, and the Yulgilbar Foundation. Numerous pharmaceutical and biotechnology companies have supported data collection and analysis. In-kind support has also been provided by Sir Charles Gairdner Hospital, CogState Ltd, Hollywood Private Hospital, the University of Melbourne, and St Vincent’s Hospital.References
1. Chételat, Gael, et al. "Accelerated cortical atrophy in cognitively normal elderly with high β-amyloid deposition." Neurology 78.7 (2012): 477-484.
2. Ayton S, Fazlollahi A, Bourgeat P, et al. Cerebral quantitative susceptibility mapping predicts amyloid-beta-related cognitive decline. Brain : a journal of neurology 2017; 140(8): 2112-9.
3. Bourgeat P, Villemagne VL, Dore V, et al. Comparison of MR-less PiB SUVR quantification methods. Neurobiology of aging 2015; 36: S159-S66.
4. Wu, Bing, et al. "Whole brain susceptibility mapping using compressed sensing." Magnetic resonance in medicine 67.1 (2012): 137-147.
5. Li W, Avram AV, Wu B, Xiao X, Liu C. Integrated Laplacian-based phase unwrapping and background phase removal for quantitative susceptibility mapping. NMR in biomedicine 2014; 27(2): 219-27.
6. Fazlollahi, Amir, et al. "A normalisation framework for quantitative brain imaging; application to quantitative susceptibility mapping." Biomedical Imaging (ISBI 2017), 2017 IEEE 14th International Symposium on. IEEE, 2017.
7. Ayton S, Faux NG, Bush AI, Alzheimer's Disease Neuroimaging I. Ferritin levels in the cerebrospinal fluid predict Alzheimer's disease outcomes and are regulated by APOE. Nature communications 2015; 6: 6760.
Figures