3771
Fluid and White Matter Suppression sequence for detecting epileptogenic zones of focal cortical dysplasia1Radiology, Xuanwu Hospital, Capital Medical University, Beijing, China, 2MR Collaborations NE Asia, Siemens Healthcare, Beijing, China, 3Advanced Clinical Imaging Technology (HC CMEA SUI DI PI), Siemens Healthcare AG, Lausanne, Switzerland, 4Radiology, University Hospital (CHUV), Lausanne, Switzerland, 5LTS5, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
Synopsis
The aim of this study was to evaluate a novel sequence, called the fluid and white matter suppression (FLAWS) sequence, for detecting focal cortical dysplasia (FCD) lesions. FLAWS provides two sets of 3D contrast images from one acquisition and subsequently calculates another set of images that can suppress the signal from both the cerebral spinal fluid and white matter. The detection rate of the FCD lesions on FLAWS was higher than on the conventional 2D MR scan and 3D fluid-attenuated inversion recovery scan. Additionally, the transmantle sign, which is widely believed to be specific for FCD type II, could also be observed in FCD type I on the FLAWS images.
Purpose
About 45% of the epileptogenic zones of focal cortical dysplasia (FCD) have negative findings on conventional MRI. The purpose of this study was to evaluate the diagnostic value of a novel sequence, called fluid and white matter suppression (FLAWS), for detecting FCD epileptogenic zones. FLAWS1 can provide two sets of 3D, high-spatial-resolution images from a single acquisition: one image suppresses the white matter signal (WM-nulled image) and the other suppresses signal from the cerebrospinal fluid (CSF-nulled image). Subsequently, a new contrast image set (based on these two image sets) is calculated, and this can suppress both the WM and CSF signals, resulting in a gray-matter-specific image (FLAWS contrast image). Because the FCD is caused by localized cortical disruptions of neuronal proliferation and organization, the FLAWS contrast have great potential for improving the visualization of epileptogenic zones. This is the first time FLAWS has been used in FCD patients.Methods
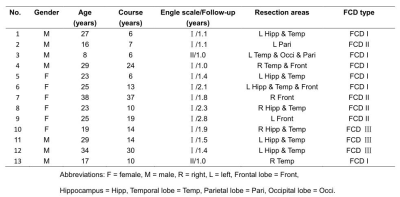
Thirteen FCD patients confirmed by pathology and with good outcomes (Class I or II, according to the Engel Epilepsy Surgery Outcome Scale) were retrospectively recruited for the study (Table 1). All the patients underwent a preoperative whole-brain MR examination on a MAGNETOM Verio 3T MR Scanner (Siemens Healthcare, Erlangen, Germany) that included conventional sequences (T2WI, T1WI, and axial/coronal FLAIR), 3D FLAIR, and FLAWS based on a prototypical sequence.
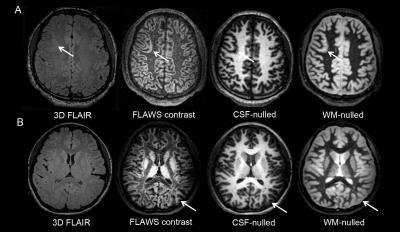
The location of the surgical resection area was regarded as the FCD epileptogenic zone. Two radiologists evaluated the detectability of all types of images through five criteria in the FCD epileptogenic zone areas2, namely: cortical thickening or thinning; abnormal cortical signal intensity; the blurred junction of the gray-white matter; abnormal signal intensity of the subcortical white matter; and the transmantle sign. Imaging was considered to be negative when none of the above five MR imaging criteria were present, and as positive when at least one of the five criteria were present. Detection rates of the conventional, FLAWS, and 3D FLAIR sequences were compared using the McNemar test.
Results
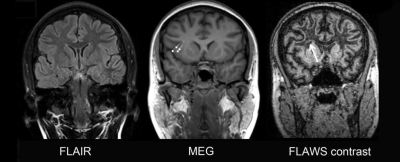
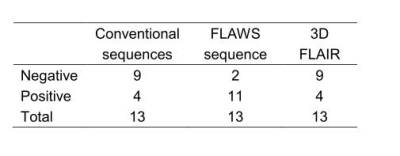
The detection rate of FLAWS was higher than that of the conventional sequences (P = 0.039) and 3D FLAIR (P = 0.07). The number of patients whose epileptogenic zones were positive on FLAWS but negative on the conventional or the 3D FLAIR sequence was 8 or 7, respectively (Table 2 and Fig.1). The most sensitive image to display five features was the FLAWS contrast image. The transmantle sign, which is widely believed to be a specific feature of FCD type II, could also be observed in FCD type I on the FLAWS sequence (Fig.2).Discussion
The advantages of FLAWS for FCD include: 1) the detection rate of FLAWS is higher than that of conventional sequences and the 3D FLAIR sequence. 2) The volumetric nature of the three images also allows for volume or voxel-based morphometric quantitative MRI analysis to further improve the detection and. And 3) because the three images derive from the same acquisition, they are perfectly registered with each other. The co-registered nature of FLAWS allows for its further usage in the integration of multimodal 3D imaging data.
One notable finding of our study was that the transmantle sign was not specific for FCD type II on FLAWS. This is not consistent with previous studies that found that the transmantle sign is a specific criterion for FCD type II on the conventional sequence2,3. It may expand the understanding of the imaging criteria and corresponding pathological basis of the transmantle sign. According to the Blümcke classification4, neocortical dyslamination exists in both FCD types I and II, whereas dysmorphic neurons and ball-on-cells only exist in types IIA and IIB, respectively. As the transmantle sign can be found in both types, its pathology is more likely related to abnormal cortical layers than to dysmorphic neurons and ball-on-cells. This inference is supported by a pathology-based study that demonstrated that various layer-specific markers were abnormally present and continuously distributed in the neurons of the transmantle dysplasia epileptogenic zone5 and by a clinical retrospective study showing that a single patient with the transmantle sign was FCD type I6.
The small sample size in our study may be the reason the difference between FLAWS and 3D FLAIR did not reach statistical significance. Further studies with larger sample sizes should be conducted.
Conclusion
FLAWS (and particularly the FLAWS contrast image) can improve the detection of FCD epileptogenic zones better than conventional sequences or 3D FLAIR. The transmantle sign is not specific for FCD type II on FLAWS.Acknowledgements
No acknowledgement found.References
1. Tanner M, Gambarota G, Kober T, et al. Fluid and white matter suppression with the MP2RAGE sequence. Journal of Magnetic Resonance Imaging : JMRI. 2012;35(5):1063-1070.
2. Bernasconi A, Bernasconi N, Bernhardt BC, Schrader D. Advances in MRI for 'cryptogenic' epilepsies. Nature Reviews Neurology. 2011;7(2):99-108.
3. Mellerio C, Labeyrie MA, Chassoux F, et al. 3T MRI improves the detection of transmantle sign in type 2 focal cortical dysplasia. Epilepsia. 2014;55(1):117-122.
4. Blümcke I, Thom M, Aronica E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52(1):158-174.
5. Sakakibara T, Sukigara S, Saito T, et al. Delayed maturation and differentiation of neurons in focal cortical dysplasia with the transmantle sign: analysis of layer-specific marker expression. Journal of Neuropathology and Experimental Neurology. 2012;71(8):741-749.
6. Wang DD, Deans AE, Barkovich AJ, et al. Transmantle sign in focal cortical dysplasia: a unique radiological entity with excellent prognosis for seizure control. Journal of Neurosurgery. 2013;118(2):337-344.
Figures