3769
ADHD and stimulant medication disrupt the temporal lag structure within default mode and salience networks1Department of Neuroscience, Brighton and Sussex Medical School, Falmer, United Kingdom, 2Clinical, Edu & Hlth Psychology, University College London, London, United Kingdom
Synopsis
Attention Deficit Hyperactivity Disorder (ADHD) is a neurodevelopmental disorder that has been associated with reduced Default Mode (DMN) (precuneus) and salience network (Anterior Cingulate) activity in conventional resting-state fMRI studies (rs-fMRI). However, these studies ignore the significant temporal structure in these data. Thirty ADHD patients and 30 matched controls underwent rs-fMRI on two occasions, 90-minutes after blindly administered stimulant medication and placebo. Using a novel ‘lag-thread’ analysis we demonstrate faster recruitment of the precuneus in ADHD which additionally correlated with severity of inattention. Stimulants slowed ACC recruitment, together suggesting potential importance of temporal activation of these regions in ADHD.
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a neurodevelopmental disorder characterised by distractibility, impulsivity and hyperactivity. Though the aetiology of ADHD remains only partially understood, genetic association with functional polymorphisms within dopamine receptors (DRD4, DRD5) and therapeutic efficacy of stimulant medications such as methylphenidate (MPH) and dexamfetamine implicate deficits in dopamine and noradrenaline signalling1. Given the widespread projections of both dopamine and noradrenergic neurons, ADHD studies have increasingly shifted from a focus on regional brain activity towards analyses of distributed brain networks. Studies to date demonstrate ADHD-associated reductions in activity within the Default Mode (DMN) (particularly the precuneus) and salience networks (notably Anterior Cingulate Cortex - ACC)2.
ACC activity is also modulated by stimulant medications; a recent meta-analysis of 14 go/no-go fMRI studies shows an association with reduced ACC and pre-SMA activity in ADHD3. The ACC forms a key node within the salience network and is implicated in shifting attention to context- and goal-relevant stimuli. It is therefore noteworthy that stimulant-induced changes in ACC reactivity have been linked to associated improvement in reward-related processing on the monetary incentive delay task4.
To date studies of ADHD have focussed exclusively on zero-lag synchronicity of brain activity. However, resting-state fMRI (rs-fMRI) contains substantial temporal structure suggesting apparent changes in connectivity at zero-lag may instead reflect a shift in temporal structure. To address this, here we use a novel lag-thread analysis5 to characterise the effects of ADHD and stimulant medication on this temporal propagation.
Methods
Thirty ADHD patients (mean 33.7±9.5 years, 19 male) and 30 (mean 32.6±9.5 years, 19 male) age- (p=0.66), IQ- (ADHD: 109.0±6.57, controls: 110.1±7.06, p=0.53) and sex-matched controls underwent rs-fMRI scanning on twice (90 minutes after blindly administered placebo/stimulant medication). All completed a battery of questionnaires and cognitive tasks including Conners Self Report Questionnaire (mean±s.d. Inattention: ADHD:26.3±5.8, controls:9.9±5.7; Hyperactivity: ADHD:24.4±6.6, controls:11.3±5.7; Impulsiveness: ADHD:23.7±7.4, controls:7.6±4.1), Tridimensional Personality Questionnaire and the Kirby delay-discounting questionnaire.
Each rs-fMRI session acquired 196 volumes on a Siemens 1.5T Avanto using a multi-echo sequence to minimise movement effects (32 channel head coil, TR=2.57s, TE=15,34,54ms, resolution=(3.8x3.8x4.2)mm3, FOV=(24cm)3), with participants fixating on a crosshair. Functional images were aligned, co-registered to a T1-weighted MPRAGE image then warped to MNI space using AFNI6. Multi-echo images were then optimally combined using ME-ICA (Multi-Echo Independent Component Analysis7) which uses Principal Component Analysis (PCA) time-series decomposition to remove non-bold-like components.
We then performed a conventional zero-lag resting state analysis using FSL MELODIC8 and a lag-thread analysis following the methods of Mitra5 to identify the optimal time-delays required to maximise correlations between voxel time-series using (6mm)3 voxels to improve signal-to-noise. PCA decomposition of the resultant time-delay matrix then identified independent ‘lag-threads’; distinct patterns of signal propagation across the brain. Finally, time delays were averaged within voxels to produce a ‘latency’, or average time delay with respect to the rest of the brain. Groups were compared using two-sample t-tests and latencies correlated with behavioural measures. Results are reported at robust whole-brain TFCE (threshold-free cluster enhanced)-corrected thresholds9.
Results
Zero-lag resting state analysis:
Conventional resting state analysis found 15 non-noise networks including the default mode and salience networks. However, at the robust thresholds reported here we did not identify statistically significant main effects of group or stimulant medication within any network.
Lag-thread analysis: We first replicated previous findings of approximately 8 independent temporospatial sequences of activity that propagate across the brain. Similar to Mitra5 these included some ‘lag threads’ originating in the brain-stem and others in higher-order cortical regions.
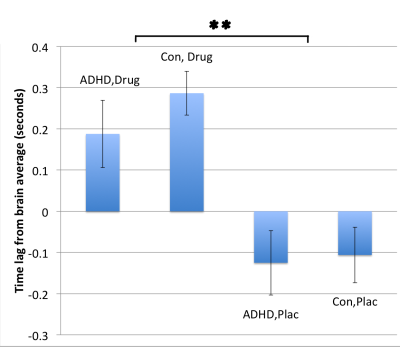
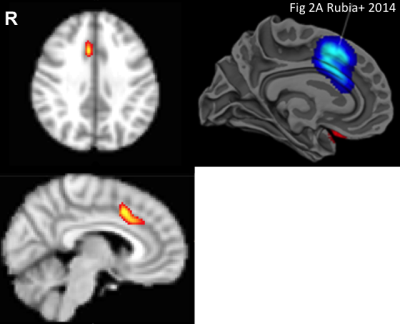
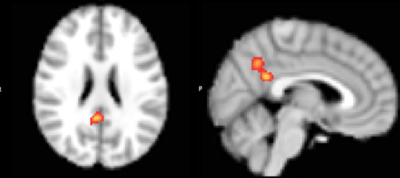
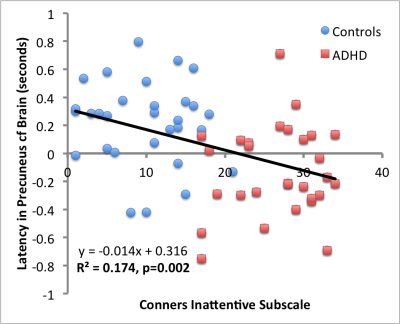
Analysis of mean latencies showed a significant main effect of stimulant medication on activation lag time within the anterior cingulate cortex (ACC) (fig. 1), slowing this region’s time of activation by a mean (±s.e.) of 0.34±0.08s compared to all other brain areas (TFCE corrected p<0.01, fig 2). In contrast, ADHD was associated with significantly quicker (mean 0.36±0.07s) recruitment of the precuneus compared to other brain regions (TFCE corrected p<0.05, fig 3). Interestingly, mean lag within this DMN region additionally correlated with ADHD participants inattention score (R2=0.174, p=0.002, fig 4).
Discussion
Here using a novel lag thread analysis we identify a specific effect of ADHD on the temporal activation of the precuneus, a key component of the DMN and a selective effect of stimulant medication on ACC, a core node in the salience network. Furthermore, temporal activation of the precuneus correlated with severity of inattention in ADHD.
These findings suggest that prior observations of ADHD and stimulant medication related effects in each of these regions in conventional zero-lag resting state analysis could (in part) be driven by a shift in the temporal recruitment of these regions.
Acknowledgements
The authors acknowledge funding from the Brighton and Sussex Medical School and the Sussex Sackler Centre.References
1. B. Franke, S. V. Farone, P. Asherson et. al. The genetics of attention deficit/hyperactivity disorder in adults, a review. Molecular Psychiatry 2012;17:960–987
2. F.X. Castellanos, D. S. Margulies, C. Kelly et. al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry, 2008;63(3):332-7
3. K. Rubia, A. A. Alegria, A. I. Cubillo et. al. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol. Psych 2014;76(8):616-628
4. L. J. S. Schweren, A. Groenman, D. von Rhein et. al. Stimulant Treatment Trajectories Are Associated With Neural Reward Processing in Attention-Deficit/Hyperactivity Disorder. J Clin Psychiatry, 2017;78(7):e790-e796
5. A. Mitra, A. Z. Snyder, T. Blazey, M. E. Raichle. Lag threads organize the brain’s intrinsic activity. PNAS 2015;112(17)E2235-E2244
6. R. W. Cox. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3)162-173
7. P. Kundu, S. J. Inati, J. W. Evans, W. M. Luh, P. A. Bandettini. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage 2012;60(3):1759-1770
8. C. F. Beckmann, S. M. Smith. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 2004;23(2):137-52
9. S. M. Smith, T. E. Nichols. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44(1):83-98
Figures