3756
Non-Invasive Imaging of Brain Clearance Pathways using Multiple Echo Time ASL: An Aquaporin-4 Study1UCL Centre for Advanced Biomedical Imaging, Division of Medicine, University College London, London, United Kingdom, 2Karolinska Institutet, Stockholm, Sweden, 3University of Oslo, Oslo, Norway, 4Neuroradiological Academic Unit, UCL Institute of Neurology, University College London, London, United Kingdom, 5Leonard Wolfson Experimental Neurology Centre, UCL Institute of Neurology, University College London, London, United Kingdom
Synopsis
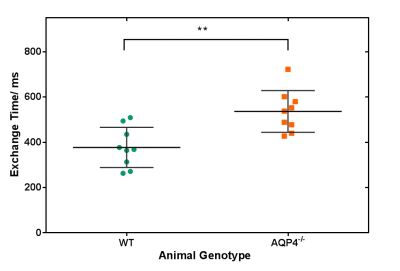
We have developed the first non-invasive technique that is able to detect changes in brain AQP4-mediated clearance pathways. Our multi-TE ASL technique measures the exchange of vascular water into cortical brain tissue of mouse brain. We report a significant increase in the cortical exchange time between WT (377 ± 89ms) and AQP4-deficient (536 ± 92ms) mice. While measured CBF, ADC and δa did not detect differences, suggesting preserved haemodynamic and energetics between groups. This highlights the novelty of the technique being targeted method to assess water transport in brain clearance pathways, to help better understand neurodegenerative diseases.
Introduction
The extracellular accumulation of protein amyloid-β (Aβ) is a hallmark of Alzheimer’s disease (AD). Studies demonstrate that impaired rates of clearance increase parenchymal Aβ accumulation in AD [1]. Brain aquaporin-4 (AQP4) water channels appear to play a key role in Aβ clearance [2-4]. This raises the exciting prospect that AQP4-mediated clearance pathways, such as the recently uncovered glymphatic system [2], represent a breakthrough target for early diagnosis and effective therapeutic intervention in AD. There is, however, currently a lack of non-invasive tools available to assess AQP4-mediated brain clearance pathways. Our previous work demonstrated the feasibility of using multiple echo time (multi-TE) ASL to assess vascular permeability to water in the mouse [5]. We hypothesise that a reduction in AQP4 water channels, that reside in the endfeet of astrocytes, will impair rates of water exchange across the blood-brain interface. Thus, our non-invasive technique will detect slower movement of labelled vascular water into the extravascular tissue of AQP4-deficient (AQP4-/-) mice. This novel approach may provide a clinically viable tool to better understand the dynamic role of AQP4 in brain protein clearance, working towards new and non-invasive biomarkers for early diagnosis of AD.Methods
Images were acquired using an Agilent 9.4T imaging system with a two-channel array surface coil (Rapid Biomedical) in ten male AQP4-/- mice [6] and ten male C57/B6 WT controls at 6 months old. Anaesthesia (~2% isoflurane in a mixture of 1.0L/min medical air) was adjusted throughout the scans to maintain the respiration rate at ~100bpm. A multi-TE ASL protocol was used, based on flow-alternating inversion recovery (FAIR) sequence with a two-shot segmented SE-EPI readout. Sequence parameters: TE = 15, 18, 23, 30, 40, 50, 65ms; TI = 1500ms; FOV = 25x25mm; matrix size = 32x32; TR = 5000ms; repetitions = 15. Arterial transit time, δa, was acquired with separate single TE, multi-TI ASL protocol. Sequence parameters: TI = 200, 300, 400, 500ms; TE = 10ms; all other parameters consistent with previous protocol. Apparent diffusion coefficient (ADC) was also estimated using a two b-value approach: b-values = 0 and 1030.5s/mm2; TE = 23.82ms; TR = 2500ms; data matrix = 64x64; repetitions = 10.
Analysis was performed on a manually defined cortical region of interest (ROI) using Matlab R2015a (Mathworks Inc.). The ASL signal intensity was evaluated using a three parameter fit to a bi-exponential model, to estimate the intravascular and extravascular ASL signal intensity weightings [5]. The ASL signal weightings were used to measure the cortical exchange time (δ – δa), derived from the two compartment kinetic perfusion model [7-9]. The exchange time indicates the time for magnetically labelled vascular water to exchange into brain tissue once labelled bolus reaches the imaging slice. CBF and δa were measured from the same ROI using the adapted perfusion model [8, 10]. The ADC was evaluated from diffusion-weighted images using the standard mono-exponential model.
Results
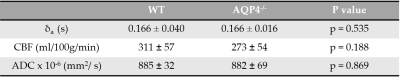
Figure 1 shows a marked difference in the mean cortical exchange time between the AQP4-/- (536 ± 92ms) and WT animals (377 ± 89ms) (p = 0.004). This reflects slower movement of vascular water into the extravascular cortical tissue in the absence of AQP4 water channels. Table 1 shows the δa, CBF and ADC from cortical region in both groups. The δa results show no detectable time difference in the arrival of vascular water at the imaging slice between animal models. Table 1 also suggests that AQP4 has no significant impact on CBF or ADC measurements.Discussion
Multi-TE ASL provides a novel technique for assessing vascular permeability to water in cortical brain tissue. Here, the exchange time measurements are able to detect marked changes in water transport due to AQP4 channels, supporting the role of AQP4 in the movement of water across the brain-blood interface [4,11]. In previous studies, evidence of a distinct phenotype of AQP4-/- animals only emerges in pathophysiological conditions [12, 13]. Accordingly, conventional CBF and ADC imaging metrics were unable to detect differences between animal cohorts, which indicates preserved cerebral haemodynamics, thus highlighting the specificity of the multi-TE ASL approach to target impairments to AQP4-mediated water exchange into the brain-blood interface.Conclusion
We have developed the first non-invasive imaging technique to assess AQP4-mediated water transport at the brain-blood interface. Previous studies suggest that the capacity of this water transport system is a key determinate of the rate of Aβ clearance from the brain. The emerging importance of the AQP4-mediated clearance pathways, such as the glymphatic system, make this technique a promising and clinically feasible tool for better understanding the role of AQP4-mediated clearance in Alzheimer’s disease.Acknowledgements
This work is supported by the Medical Research Council (MR/K501268/1), the EPSRC-funded UCL Centre for Doctoral Training in Medical Imaging (EP/L016478/1) and the UCL Leonard Wolfson Experimental Neurology Centre (PR/YLR/18575), together with the Wellcome Trust and Royal Society.References
[1] Mawuenyega, K.G., et al., Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science, 2010. 330(6012): p. 1774.
[2] Iliff, J.J., et al., A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid Science Translational Medicine, 2012. 4(147): p. 147ra111-147ra111.
[3] Xu, Z., et al., Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Molecular Neurodegeneration, 2015. 10(1): p. 58.
[4] Nagelhus, E.A. and O.P. Ottersen, Physiological Roles of Aquaporin-4 in Brain. Physiological Reviews, 2013. 93(4): p. 1543-1562.
[5] Ohene, Y., et al., Non-invasive Assessmen tof Vascular Water Permeability in the Mouse Brain using multi-TE ASL Proc 25th Intl Soc Mag Reson Med, 2017: p. 0475.
[6] Thrane, A.S., et al., Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc Natl Acad Sci U S A, 2011. 108(2): p. 846-51.
[7] Wells, J.A., et al., Measuring Biexponential Transverse Relaxation of the ASL Signal at 9.4 T to Estimate Arterial Oxygen Saturation and the Time of Exchange of Labeled Blood Water into Cortical Brain Tissue. Journal of Cerebral Blood Flow & Metabolism, 2013. 33(2): p. 215-224.
[8] Buxton, R.B., et al., A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med, 1998. 40(3): p. 383-96.
[9] Alsop, D.C. and J.A. Detre, Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab, 1996. 16(6): p. 1236-49.
[10] Wegener, S., et al., Quantification of rodent cerebral blood flow (CBF) in normal and high flow states using pulsed arterial spin labeling magnetic resonance imaging. J Magn Reson Imaging, 2007. 26(4): p. 855-62.
[11] Filippidis, A., R. Carozza, and H. Rekate, Aquaporins in Brain Edema and Neuropathological Conditions. International Journal of Molecular Sciences, 2016. 18(1): p. 55.
[12] Amiry-Moghaddam, M. and O.P. Ottersen, The molecular basis of water transport in the brain. Nature Reviews Neuroscience, 2003. 4(12): p. 991-1001.
[13] MacAulay, N. and T. Zeuthen, Water transport between CNS compartments: contributions of aquaporins and cotransporters. Neuroscience, 2010. 168(4): p. 941-956.
Figures