3754
Semiautomatic Quantification of Cortical Amyloid with 18F-florbetaben PET/MRI in Alzheimer's disease and Other Neurodegenerative Disorders1Radiology, Yeouido St. Mary's Hospital, The Catholic University of Korea, Seoul, Republic of Korea, 2Radiology, Stanford University, Stanford, CA, United States, 3Neurology and Neurological Sciences, Stanford University, Stanford, CA, United States
Synopsis
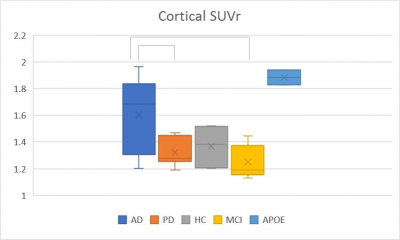
Amyloid PET is a useful quantitative biomaker in patients with dementia. We compared cortical amyloid deposition in Alzheimer’s disease (AD), Parkinson’s disease (PD), mild cognitive impairment (MCI) and cognitively normal elderly subjects and correlated it with cortical CBF and volume using simultaneous 18F-florbetaben PET/MRI. Total cortical SUV is significantly higher in AD than PD and MCI. Precuneus, superior temporal, and calcarine cortices were identified as significant regions on amyloid PET to discriminate between AD and other neurodegenerative diseases.
Introduction
Alzheimer’s disease (AD) is an age-related neurodegenerative disease and the leading cause of dementia in the elderly. Beta-amyloid plaque (Aβ) accumulation in brain cortex is a pathological hallmark of AD.1 Reduced cerebral blood flow (CBF) and cortical volume are also known features of AD2, 3, but fewer studies have directly measured amyloid deposition with brain structural and physiologic changes in the same subjects.4-6 The purpose of this study is to compare cortical amyloid deposition in patients with AD, Parkinson’s disease (PD), mild cognitive impairment (MCI) and cognitively normal elderly subjects. We also investigate how cortical amyloid accumulation relates to perfusion and cortical volume changes in the same subjects.
Methods
28 subjects (8 AD, 7 PD, 6 healthy controls (HC), 5 MCI, and 2 cognitively normal APOE-E4 homozygous (APOE) subjects) with a mean age of 67.5±8.1 years (15/13 male/female) underwent 18F-florbetaben PET/MRI (Signa, GE Healthcare). Pseudocontinuous ASL was acquired in 23 subjects. All PET and ASL CBF images were registered to the MNI brain template using ANTS software.7 Within cortical regions of interests (ROIs) defined by the MNI atlas, supratentorial cortical standardized uptake value ratio (cSUVr) was calculated using cerebellar gray matter as a reference region.8, 9 Cortical CBF was calculated from ASL in the same predefined cortical ROIs. Cortical volume was measured from a T1-weighted image using FreeSurfer (v 6.0) software (http://surfer.nmr.mgh.harvard.edu/).
Results
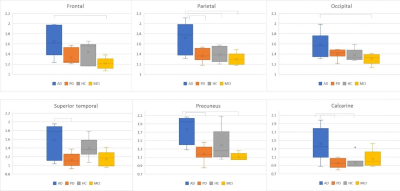
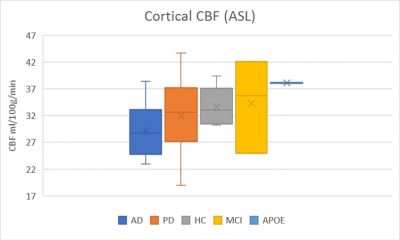
The age of HC (mean 70.1) was slightly older than AD (mean 65.5) and PD (mean 64.8), but was not statistical significant. Mean cSUVr of 18F-florbetaben PET images was significantly higher in AD (1.6±0.28) than PD (1.32±0.10, p=0.045) and MCI (1.24±0.12, p=0.019). The cSUVr was significantly higher in AD than MCI in frontal, parietal, and occipital regions; cSUVr was significantly higher in AD compared to PD in calcarine, precuneus, and superior temporal regions (p < 0.05). The parietal cSUVr was significantly higher in AD compared to PD, HC, and MCI groups (p<0.05). Two cognitively normal APOE-E4 homozygous subjects showed high cSUVr (1.88±0.08). Cortical CBF from ASL, cortical volumes, and hippocampal volumes were slightly lower in AD than PD, MCI and HC without statistical significance. There was no significant correlation cSUVr with CBF and cortical volume.
Discussion
This semiautomatic 18F-florbetaben PET/MRI quantification method is a useful method to differentiate AD from other neurodegenerative disease. Precuneus, superior temporal, and calcarine cortices were identified to be significant regions on amyloid PET for discrimination between AD and other diseases and the parietal region was the most discriminative area for AD. While there was no significant difference in cSUVr between AD and HC, the older age of HC is likely a confounder and requires further studies. The cognitively normal APOE-E4 homozygous subjects showed high cSUVr without reduced cortical CBF and cortical atrophy. These findings suggest that substantial amyloid deposition in cortical areas has already occurred before CBF alteration and cortical volume change.10 Compared with CBF alteration and cortical volume change, the semiautomatic quantification of amyloid PET may be more sensitive tool to detect the early AD and preclinical AD in clinical practice.
Acknowledgements
We would like to acknowledge support of the Stanford Alzheimer’s Disease Research Center (ADRC).References
1. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82:239-259.
2. Bangen KJ, Clark AL, Edmonds EC, et al. Cerebral Blood Flow and Amyloid-beta Interact to Affect Memory Performance in Cognitively Normal Older Adults. Frontiers in aging neuroscience. 2017;9:181.
3. Wierenga CE, Hays CC, Zlatar ZZ. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2014;42 Suppl 4:S411-419. 4. Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of neurology. 2004;55:306-319.
5. Tosun D, Schuff N, Jagust W, Weiner MW. Discriminative Power of Arterial Spin Labeling Magnetic Resonance Imaging and 18F-Fluorodeoxyglucose Positron Emission Tomography Changes for Amyloid-beta-Positive Subjects in the Alzheimer's Disease Continuum. Neuro-degenerative diseases. 2016;16:87-94.
6. Ruan Q, D'Onofrio G, Sancarlo D, Bao Z, Greco A, Yu Z. Potential neuroimaging biomarkers of pathologic brain changes in Mild Cognitive Impairment and Alzheimer's disease: a systematic review. BMC geriatrics. 2016;16:104.
7. Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). NeuroImage. 1995;2:89-101.
8. Joshi AD, Pontecorvo MJ, Lu M, Skovronsky DM, Mintun MA, Devous MD, Sr. A Semiautomated Method for Quantification of F 18 Florbetapir PET Images. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015;56:1736-1741.
9. Blautzik J, Brendel M, Sauerbeck J, et al. Reference region selection and the association between the rate of amyloid accumulation over time and the baseline amyloid burden. European journal of nuclear medicine and molecular imaging. 2017;44:1364-1374.
10. Lim YY, Ellis KA, Pietrzak RH, et al. Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology. 2012;79:1645-1652.
Figures
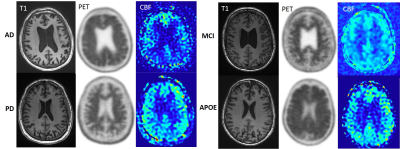
Figure 1. T1-weighted, 18F-florbetaben PET, and arterial spin labeling(ASL) CBF images of Alzhemier’s disease(AD), Parkinson’s disease(PD), mild cognitive impairment (MCI) and cognitively normal APOE-E4 homozygous subject(APOE). PET scans demonstrate cortical uptake in AD and APOE. ASL CBF decreased in both frontal and parietal regions in AD, consistent with typical features of AD.