3752
Apolipoprotein E e4 allele is associated with higher structural brain changes in patients with mild cognitive impairment1Division of Translational Medicine,Research Branch, Sidra Medicine, Doha, Qatar, 2American School, Doha, Qatar, 3Diagnostic Imaging, Sidra Medicine, Doha, Qatar, 4Center for Magnetic Resonance and Optical Imaging, Radiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
The aim of this study was to measure the difference in cortical thickness, subcortical volume and CSF biomarkers between MCI patients who carry or lack ApoE ε4 allele. High-resolution T1-weighted images were used for the measurement of cortical thickness and subcortical volume using FreeSurfer. MCI patients who carry ApoE ε4 showed significant reduction in cortical thickness and subcortical volume in multiple brain regions than non-carriers. This suggests that having an ApoE ε4 allele could be a risk factor for the larger tissue damage in the brain of MCI, and these patients may have higher chance of developing Alzheimer’s or other dementia.
Introduction:
Apolipoprotein E (ApoE) ε4 allele is known risk factor for Alzheimer disease and other neurological disorders 1,2. Mild cognitive impairment (MCI), an intermediate stage of cognitive decline, have an increased risk of developing Alzheimer or other dementia. The purpose of this study was to measure differences in cortical thickness and subcortical volume and cerebrospinal fluid (CSF) biomarkers among MCI patients who carry or lack ApoE ε4 allele. Association between CSF biomarkers and cortical and subcortical changes was also evaluated.Materials and methods:
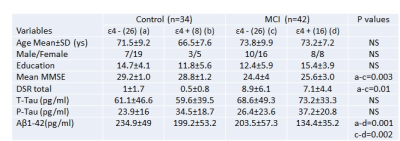
Institutional regulatory board and ethics committee approved the study protocol. With informed consent, 34 healthy control and 42 MCI (table 1) underwent clinical assessment including Mini-mental state examination (MMSE) and whole brain MRI on 1.5-T clinical MR scanner (Siemens Medical Systems, Malvern, PA, USA). MR imaging including T2- and T1-weighted imaging and high-resolution T1-weighted 3D volumetric MPRAGE (parameters: TR/TE = 3,000 ms/3.5 ms, slice thickness = 1.2 mm, number of slice=160, FOV of 240×240 cm2 and 192 phase encode steps, and flip angle = 8°) was performed. Both T1-, and T2-weighted images were examined for any gross brain pathology such as cysts, tumors, or any other mass lesions and presence of such anomaly was used as an exclusion criteria. High-resolution T1-weighted images were used for measurement of cortical thickness and subcortical volume using FreeSurfer software. The age and gender were included as covariates in the analysis. Monte Carlo simulations with 10,000 iterations were applied to correct for multiple comparisons using a cluster-wise threshold of p < 0.05 3. All the statistical computations were performed using the Statistical Package for Social Sciences 16. Demographic, neurocognitive performance and subcortical volumes were assessed by Chi-square and independent student samples t-test. Pearson's correlation was performed for the correlation analysis. Total -tau (t-tau), phosphorylated-tau (p-tau), and beta-amyloid 1-42 (Ab1-42) were quantified form CSF using method described previously 4.Results:
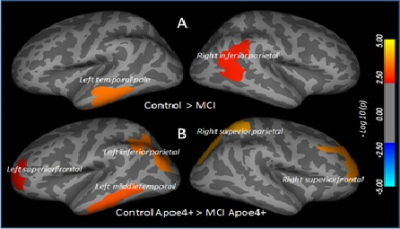
No significant difference in demographic parameters was observed among groups. No substantial difference in cortical thickness was observed between ApoE ε4+ and ApoE ε4- subjects either for control or MCI. However, ApoE ε4+ control showed higher cortical thickness (non-significant) than ApoE ε4- control and ApoE ε4+ MCI showed lower cortical thickness (non-significant) than ApoE ε4- MCI. Overall, MCI showed significantly lower cortical thickness in the left temporal pole and right inferior parietal (Figure 1) than control. Subcortical volume was significantly decreased in the left and right hippocampus, left and right accumbens, right pallidum, corpus collosum in MCI as compared to control. ApoE ε4+ MCI showed significantly reduced cortical thickness in the left and right superior frontal, left inferior and right superior parietal, and left middle temporal as compared to Apoe ε4+ control, and significantly deceased bilateral hippocampal and right accumbens volume as compared to ApoE ε4- control. Right pallidum and corpus callosum volumes were significantly lower in ApoE ε4- MCI than ApoE ε4- control. ApoE ε4+ MCI showed significantly lower value of Luminex Aβ1-42 in CSF than ApoE ε4- MCI. Luminex Aβ1-42 was significantly correlated with cortical thickness in the right superior parietal in ApoE ε4+ MCI, while it correlated bilaterally with hippocampus and accumbens volumes in ApoE ε4- MCI.Discussion:
Reduced cortical thickness and subcortical volume were observed in multiple brain regions of MCI. ApoE ε4+ MCI showed greater reduction in cortical thickness and subcortical volume than non-carriers. This suggests that the presence of ApoE ε4 allele in MCI could be a risk factor for the larger tissue damage in the brain. Presence of significant association of brain tissue changes with CSF Luminex Aβ1-42 suggest that amyloid play a role in the pathology. This observation is supported by the previous immunohistopathological studies showing significantly higher deposition of amyloid plaques and neurofibrillary tangles in the brain of MCI patients with ApoE ε4 carriers than non-carriers 5. The current study provide a noninvasive method to see the effect of ApoE ε4 allele on the structural brain changes and may help assessing the different therapeutic interventions apply to stop or reverse these brain changes.Acknowledgements
Sidra Medicine provides the work station to process the MRI data.References
1- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc Natl Acad Sci USA. 2006;103:5644–51.
2- Cosentino S, Scarmeas N, Helzner E, et al. APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology. 2008;70:1842–9.
3- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999; 9(2):179–194.
4- Vanderstichele H, Bibl M, Engelborghs S, et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: A consensus paper from the Alzheimer's Biomarkers Standardization Initiative .Alzheimers Dement. 2012; 8:65-73.
5- Sabbagh MN, Malek-Ahmadi M, Dugger BN, et al. The influence of Apolipoprotein E genotype on regional pathology in Alzheimer's disease. BMC Neurol. 2013; 11:13-44.
Figures