3745
Evaluation of the Full Z Spectrum Obtained from Demented Patients1Radiology, Kyung Hee Univ. Hospital at Gangdong, Seoul, Republic of Korea, 2Biomedical Engineering, Kyung Hee University, Suwon, Republic of Korea, 3Neurology, Kyung Hee Univ. Hospital at Gangdong, Seoul, Republic of Korea
Synopsis
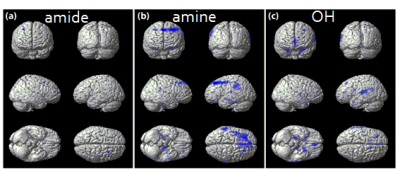
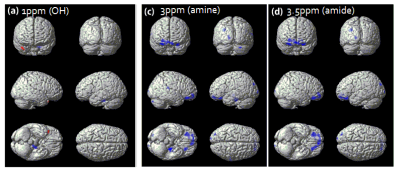
To evaluate the characteristics of chemical exchange proton pools using a CEST MRI technique in dementia patients, 19 demented and 22 non-demented subjects were scanned a full Z spectrum data using a 3D GRASE sequence. The 6 pool maps with the Lorentzian fitting and MTR asymmetry map pool were calculated and compared between the two groups. The amide, amine, hydroxyl, DWS, NOE, MT, and MTR asymmetry values at 1 ppm, 3ppm, and 3.5 ppm were higher in the demented group than in the non-demented group.
Background
Alzheimer's disease is main cause of dementia. Pathology is related with senile plaques by amyloid beta proteins and neurofibrillary tangles by tau proteins. However, there is no standard MRI imaging method to measure these proteins. Chemical exchange dependent saturation transfer (CEST) MRI is sensitive to solid-like proteins and may detect mobile proteins and peptides in tissues (Oh JH, et al. 2017). The basic principle of CEST is that if various protons are saturated, then chemical exchange happens. The exchange is closely related with proton concentration and pH in the tissue. Higher proton concentration makes higher proton transfer value and it means higher CEST signal. There was one previous study about amide protons in Alzheimer’s disease (AD) subjects (Wang R, et al., 2015). However, there is no study for investigating various substances such as amine, hydroxyl protons with a full Z spectrum. Therefore, the purpose of our study is to evaluate the characteristics of chemical exchange proton pools using a CEST MRI technique in dementia patients.Materials and Methods
Nineteen demented (mean=77.9 years, range=55-92 years) and 22 non-demented (mean=66.7 years, range=51-83 years) subjects were included in this study. MR imaging was performed using two 3.0 Tesla MRI systems. The full Z spectrum data were acquired with using a 3D gradient and spin-echo (GRASE) sequence (Zhu et al., 2010) in the brain using a 32 channel SENSE array coil. To induce saturation exchange transfer of protons, we used the following parameters: the B1 amplitude of the saturation pulse was 2T; the saturation pulse duration was 200 ms with 10 ms interval between the pulses; a number of saturation pulses were 4. Therefore, the total saturation length was 0.84 sec. We obtained the full Z spectrum by total 38 dynamics from -5.00 ppm to 5.00 ppm frequency offset ranges, using alternative increased frequency interval of 0.25 ppm from ± 0.25 ppm to ± 4.00 ppm, after that ± 4.5 ppm and ± 5.0 ppm. The first acquired image was the reference image at -40 ppm and the second acquired image was at 0 ppm offset to direct saturation of water. The scan time was 10 min 8 sec. The full Z spectrum data were analyzed by Matlab software (Mathworks, Natick, MA, USA) to map the voxel-based exchangeable signals with the special frequency offsets of protons as well as the magnetization transfer ratio (MTR) asymmetry (MTRasym) with the following steps. We quantified exchangeable protons with the Lorentzian fitting method using following formula 6 pool model consist of amide, amine, direct water saturation (DWS), nuclear overhauser effect (NOE) and magnetization transfer (MT) (Zaiss et al., 2011). The voxel-based (MTRasym maps were calculated at the frequency offsets of 1.00 ppm (hydroxyl), 3.00 ppm (amine), 3.50 ppm (amide). The two sample t-test was used for comparison between two groups.Results
Age was not significantly different between the two groups (p > 0.1693). Gender and Korean version of the Mini Mental State Examination (K-MMSE) were significantly different (p = 0.0158, p < 0.001). The amide, amine, and hydroxyl were higher in the demented group than in the non-demented group at the frontal, parietal, and temporal areas. DWS, NOE, and MT were also higher in the demented group than in the non-demented group in most of brain areas. MTR asymmetry values at 1 ppm, 3ppm, and 3.5 ppm showed higher in the demented subjects than the non-demented subjects in the frontal, temporal, and occipital area.Discussion
For diagnosis of AD, amyloid PET is the currently representative method. However, it has some disadvantages such as radiation exposure and a low spatial resolution. CEST MRI has several advantages compared with amyloid PET such as no radiation exposure, better spatial resolution, less expensive, and less time consuming. There was one previous study on dementia using CEST MRI (Wang R, et al., 2015). It showed amide proton transfer (APT) was higher in hippocampus of AD patients than the control group, but there was no statistically significant difference between AD patients and the control group in other brain structures other than hippocampus. In our study, in addition to high APT in bilateral hippocampi, proton transfer of various materials is highly expressed in various brain areas.Conclusion
In the demented subjects, amide, amine, hydroxyl values as well as MTR asymmetry values were increased, probably related with increased proteins, neurotransmitters, or metabolites such as myo-inositol in the demented patients. DWS, NOE and MT were also increased in demented group it may be related with brain tissue atrophy. Therefore, CEST MRI may be useful to investigate brain changes in demented patients without radiation exposure.Acknowledgements
The research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2014R1A2A2A01002728) and by the Convergence of Conventional Medicine and Traditional Korean Medicine R&D program funded by the Ministry of Health & Welfare through the Korea Health Industry Development Institute (KHIDI) (HI16C2352).References
JH Oh, HG Kim, DC Woo, HK Jeong, SY Lee, GH Jahng. Chemical-Exchange-Saturation-Transfer Magnetic Resonance Imaging to Map Gamma-Aminobutyric Acid, Glutamate, Myoinositol, Glycine, and Asparagine: Phantom Experiments. Journal of the Korean Physical Society, 2017:70(5):545-553.
Wang R, Li SY, Chen M1, Zhou JY, Peng DT, Zhang C, Dai YM. Amide Proton Transfer Magnetic Resonance Imaging of Alzheimer’s Disease at 3.0 Tesla: A Preliminary Study. Chin Med J (Engl). 2015:5;128(5):615-9.
Zhu, H., Jones, C.K., van Zijl, P.C., Barker, P.B., Zhou, J., 2010. Fast 3D chemical exchange saturation transfer (CEST) imaging of the human brain. Magn Reson Med 64, 638-644.
Zaiss, M., Schmitt, B., Bachert, P., 2011. Quantitative separation of CEST effect from magnetization transfer and spillover effects by Lorentzian-line-fit analysis of z-spectra. J Magn Reson 211, 149-155.
Figures