3740
Quantitative Susceptibility Mapping of post-mortem ALS brains at 7T1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Clinical Neurology, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 3Department of Radiology, University of Chicago, Chicago, IL, United States
Synopsis
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease of the motor system and its wider cortical connections. Progress in therapeutic development in ALS is compromised by a lack of specific biomarkers. In this work, we describe a platform for acquiring QSM data in post-mortem brains and propose a protocol for post-mortem QSM reconstruction. Preliminary results have shown that ALS brains had substantially greater mean susceptibility in motor cortex than control brains, which indicates that QSM has the potential to accurately quantify iron concentration and thus serve as an imaging biomarker for ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive and fatal neurodegenerative disease of the motor system and its wider cortical connections [1]. Progress in therapeutic development in ALS is compromised by a lack of specific biomarkers. Abnormal accumulation of iron in the motor cortex of ALS patients has been demonstrated by several studies [2], yet the contribution of iron overload to pathology remains unclear. Quantitative susceptibility mapping (QSM) is a novel technique that has been demonstrated to have better accuracy in quantifying cerebral iron concentration than other MR methods [3]. In this work, we describe a platform for acquiring QSM data in post-mortem brains that will later be interrogated with quantitative iron histopathology, presenting preliminary results in two ALS and three control brains that are part of a larger study.Methods
Brain sample preparation
Brains were immersion fixed in formalin after autopsy. Before MR scans, brain samples were removed from formalin and packed in a brain-shaped container filled with 3MTM Fluorinert (FC-3283), a magnetic susceptibility matched liquid. Diffusion and structural scans were additionally acquired using protocols described by Foxley et al. [4].
Data acquisition
Two ALS and three control brains were scanned at 7T (Siemens, 1Tx/32Rx coil) using a 3D multi-echo GRE sequence. Imaging parameters were: TR=38ms, TE=2ms, ΔTE=6.6ms, number of echoes=6, flip angle=15°, pixel bandwidth=651Hz, voxel size=0.5×0.5×0.55mm3.
QSM reconstruction
1. Datasets corresponding to the individual 32 channels of the head coil were exported from the scanner, with phase offsets removed via a complex division of the multi-echo phase data by the TE=2ms dataset. A coil-combined dataset was generated for each individual echo via the sum of the complex signal from the offset-corrected individual channels.
2. Phase images from 2nd to 6th echoes were unwrapped individually with a Laplacian unwrapping algorithm [5]. The eddy current induced phase component was then estimated and removed through 3D linear fitting of the unwrapped phase images. Finally, the local field variation (ΔB) map was estimated from the unwrapped phase using a voxel-wise linear fit of the 2nd to 6th echoes with respect to the TE.
3. The background field was removed using the variable-kernel sophisticated harmonic artifact reduction for phase data (V-SHARP) algorithm [6], with a maximal kernel size of 12mm and regularisation parameter of 0.02mm-1. The susceptibility maps were subsequently generated using the truncated k-space division algorithm with a threshold of 0.2 [7].
Quantitative analysis
Grey-white matter segmentation using FAST [8] was performed based on mean diffusivity maps obtained from the diffusion datasets, which exhibit strong contrast in post-mortem tissue. The motor cortex and visual cortex masks were generated from the Juelich atlas [8, 9] and co-registered to the magnitude image space using ANTs [10]. Finally, the mean susceptibility values (in parts per billion) were extracted from motor cortex and visual cortex (the latter serving as a reference).
Results
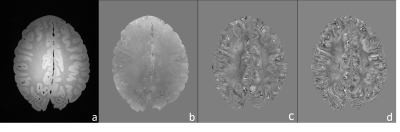
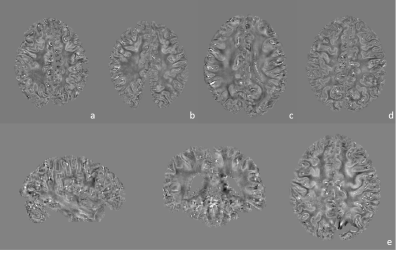
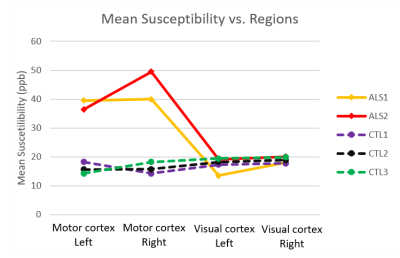
Representative magnitude and processed phase images (covering the motor cortex) are shown in Figure 1. Figure 2 shows the obtained quantitative susceptibility maps from the five post-mortem brain samples. As shown in Figure 3, the quantitative analysis indicates that the two ALS brains have substantially greater mean susceptibility within the motor cortex (41.4 ppb) than the three control brains (16.1 ppb). ALS and control brains showed similar mean susceptibility values in visual cortex (17.7 ppb and 18.7ppb, respectively), the chosen control region where relatively little neuropathology is expected.Conclusions
Our preliminary results suggest that the proposed protocol is promising for post-mortem QSM. In this work, ALS brains were found to have substantially greater mean susceptibility in motor cortex than control brains, which agrees with a previous in-vivo study [11]. This susceptibility difference could be related to disease pathology. QSM has the potential to accurately quantify iron concentration and thus serve as an imaging biomarker for ALS. Future work will optimize the phase processing protocol for the acquired post-mortem datasets, including phase unwrapping, background field removal and QSM reconstruction algorithms. These data are part of a larger study that aims to collect multi-modal MRI data in a much larger cohort of ALS and control brains.Acknowledgements
No acknowledgement found.References
1. Kiernan, Matthew C., et al. "Amyotrophic lateral sclerosis." The Lancet 377.9769 (2011): 942-955.
2. Eskreis‐Winkler, Sarah, et al. "The clinical utility of QSM: disease diagnosis, medical management, and surgical planning." NMR in Biomedicine 30.4 (2017).
3. Haacke, E. Mark, et al. "Quantitative susceptibility mapping: current status and future directions." Magnetic resonance imaging 33.1 (2015): 1-25.
4. Foxley, Sean, et al. "Improving diffusion-weighted imaging of post-mortem human brains: SSFP at 7T." Neuroimage 102 (2014): 579-589.
5. Schofield, Marvin A., and Yimei Zhu. "Fast phase unwrapping algorithm for interferometric applications." Optics letters 28.14 (2003): 1194-1196.
6. Özbay, Pinar Senay, et al. "A comprehensive numerical analysis of background phase correction with V‐SHARP." NMR in Biomedicine 30.4 (2017).
7. Haacke, E. M., et al. "Susceptibility mapping as a means to visualize veins and quantify oxygen saturation." Journal of Magnetic Resonance Imaging 32.3 (2010): 663-676.
8. Jenkinson, Mark, et al. "Fsl." Neuroimage 62.2 (2012): 782-790.
9. Eickhoff, Simon B., et al. "A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data." Neuroimage 25.4 (2005): 1325-1335.
10. Avants, Brian B., et al. "The Insight ToolKit image registration framework." Frontiers in neuroinformatics 8 (2014).
11. Schweitzer, Andrew D., et al. "Quantitative susceptibility mapping of the motor cortex in amyotrophic lateral sclerosis and primary lateral sclerosis." American journal of roentgenology 204.5 (2015): 1086-1092.
Figures