3739
Structural remodeling of the sciatic nerve differs between painful and painless diabetic polyneuropathy: an in vivo study using magnetic resonance neurography.1Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany, 2Endocrinology, Heidelberg University Hospital, Heidelberg, Germany, 3Experimental Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany, 4Neuroradiology, Würzburg University Hospital, Würzburg, Germany
Synopsis
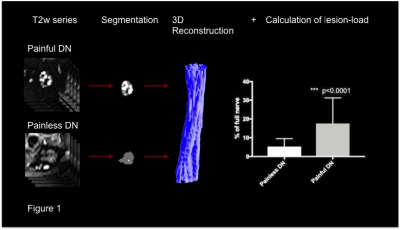
Diabetic polyneuropathy (DN) is one of the most severe complications of diabetes. It is yet uncertain why patients either suffer from painful (PDN) or painless (NPDN) diabetic polyneuropathy. We prospectively performed magnetic resonance neurography of the sciatic nerve in 120 patients suffering from DN of varying severity and correlated the results with clinical symptoms and electrophysiological data. We found a higher load of lesions to the sciatic nerve in PDN compared to PDN (p<0.0001), extending over longer distances (p<0.0001). This indicates that proximal nerve damage is one of the main contributors to the development of PDN.
Introduction:
Distal symmetric diabetic polyneuropathy (DN) is one of the most severe and yet most poorly understood complications of diabetes.1 About 30-50% of all adult diabetic patients are affected with high morbidity and healthcare costs.2 It is still unknown why some patients develop painful neuropathic symptoms (painful diabetic neuropathy, PDN) whereas others suffer from painless diabetic neuropathy (NPDN).1,3 Contrary to previous belief, recent studies have shown that the earliest signs of structural changes in neural tissue leading to DN do not occur at the level of the distal neural fibers of the feet but on the level of the distal sciatic nerve.4,5,6 The aim of this study was to detect differences in structural remodeling of the sciatic nerve in patients suffering from PDN and NPDN.
Methods:
We compared a series of 24 T2-weighed, fat-saturated axial slices (relaxation-time 5970ms, echo-time 55ms, field of view 160mm x 160mm) of the distal sciatic nerve in 120 patients suffering from DN of varying severity. Neural lesions were segmented in a semi-automatic approach with custom-written code in Fiji and Matlab. We performed a 3D-reconstruction of the lesion load in order to compare the extent and spatial distribution of lesions among patients. Results were correlated with electrophysiological parameters and neuropathy-specific clinical scores.Results:
We found a significantly higher lesion-load (p<0.0001) and -length (p<0.0001) of the distal sciatic nerve in PDN compared to NPDN. We could further show that there is an increase of lesions according to the severity of disease-specific symptoms and a significant correlation of percentage lesion-load and electrophysiological parameters associated with disease deterioration.Discussion
To our knowledge, this is the first study to visualize differences in the structural remodeling of the sciaticc nerve in patients suffering from PDN and NPDN in vivo. Differences in lesion-load and –length indicate that disease-associated pain is caused to a larger extent by structural remodeling processes in the proximal neural fibers of peripheral nerves.Conclusion:
The localization and quantification of neural lesions contributing to PDN may help to understand the pathophysiological mechanisms underlying the development of neuropathic symptoms in DN. Further, in patients suffering from DN magnetic resonance neurography may serve as an objective parameter for the evaluation of disease specific treatments.Acknowledgements
This study was funded by the German Research Council (DFG, SFB 1158)References
1. Nawroth PP, Bendszus M, Pham M, et al. The quest for more research on painful diabetic neuropathy [Internet]. Neuroscience 2017;[cited 2017 Oct 15 ] Available from: http://www.ncbi.nlm.nih.gov/pubmed/28942323
2. Alleman CJM, Westerhout KY, Hensen M, et al. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: A review of the literature [Internet]. Diabetes Res. Clin. Pract. 2015;109(2):215–225.Available from: http://linkinghub.elsevier.com/retrieve/pii/S0168822715002296
3. Spallone V, Greco C. Painful and painless diabetic neuropathy: One disease or two? Curr. Diab. Rep. 2013;13(4):533–549.
4. Pham M, Oikonomou D, Bäumer P, et al. Proximal neuropathic lesions in distal symmetric diabetic polyneuropathy: Findings of high-resolution magnetic resonance neurography [Internet]. Diabetes Care 2011;34(3):721–723.Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3041214&tool=pmcentrez&rendertype=abstract
5. Pham M, Oikonomou D, Hornung B, et al. Magnetic resonance neurography detects diabetic neuropathy early and with Proximal Predominance. [Internet]. Ann. Neurol. 2015;78(6):939–48.[cited 2016 Jan 18 ] Available from: http://www.ncbi.nlm.nih.gov/pubmed/26381658
6. Vaeggemose M, Pham M, Ringgaard S, et al. Magnetic Resonance Neurography Visualizes Abnormalities in Sciatic and Tibial Nerves in Patients with Type 1 Diabetes and Neuropathy [Internet]. Diabetes 2017;db161049.[cited 2017 Apr 27 ] Available from: http://www.ncbi.nlm.nih.gov/pubmed/28432188