3734
Vertex-based thalamus morphometry and tract-based spatial statistics of the white matter show differences between patients with chronic low back pain and fibromyalgiaHuiling Peng1, Alyssa Smith1, Kelly Boland1, and Jason Craggs1
1University of Missouri, Columbia, MO, United States
Synopsis
Chronic low back pain (CLBP) is now considered a disease of the central nervous system. Two-thirds of individuals with CLBP also have fibromyalgia (FM) which is a chronic pain syndrome characterized by widespread deep musculoskeletal pain and cognitive deficits. The aim of this study was to investigate gray and white matter changes between FM and CLBP groups using vertex analysis of thalamus and tract-based spatial statistics (TBSS). Significant surface depression was detected in right thalamus of patients with FM compare to CLBP. TBSS analysis showed significantly reduced FA in several white matter tracts of patients with FM compared to CLBP.
Introduction
Low back pain (LBP) is the leading cause of disability worldwide1 with approximately 10% of LBP patients develop chronic low back pain (CLBP). Despite the lack of clear pathology in CLBP, it is now considered a disease of the central nervous system (CNS) 2. Studies using magnetic resonance imaging (MRI) have reported volumetric change in gray matter (GM) and white matter (WM) of CLBP at cortical and subcortical levels compared to healthy controls 3-5. More recent studies utilizing diffusion tensor imaging (DTI) methods have reported an altered WM integrity, as measured via fractional anisotropy (FA), in disabled compared to non-disabled CLBP, and in pre-treatment compared to post-treatment CLBP 6-7. Meanwhile, as many as two-thirds of individuals with CLBP also have fibromyalgia (FM) which is a chronic pain syndrome characterized by widespread deep musculoskeletal pain and cognitive deficits 8. Studies suggest that FM is associated with structural changes in the CNS including part of the somatosensory system and part of the motor system 9. Understanding the distinctive neural mechanisms between CLBP and FM is crucial for clinical diagnosis and treatment. Therefore, the aim of this study was to investigate GM and WM changes in individuals with FM compared to individuals with CLBP. Specifically, studies have suggested that the thalamus is an important structure that mediates different components of pain: sensory discriminative (later pain pathway) and affective-motivational (medial pain pathway) components 10-11. Given the important role of thalamus as a relay between spinal and cortical structures, we examine in this study changes in thalamic morphometry via semi-automated MRI-based segmentation. Tract-based spatial statistics (TBSS, FSL) was also used to perform an extensive examination of white matter fiber tract-specific changes between FM and CLBP groups.Methods
A sample of 48 individuals with CLBP and 22 FM individuals of similar age participated in this study. A 3T Siemens Trio MRI scanner with a standard 8-channel head coil (Erlangen, Germany) was used to obtain high-resolution T1-weighted structural images and single-shot spin-echo echo-planar DTI (SE-EPI-DTI) images of the whole brain. Following acquisition, automated segmentation of the thalamus was performed on T1-weighted images using FIRST, FMRIB’s Integrated Registration and Segmentation Tool 12. Vertex analysis software then estimates thalamic shape and size with fixed number of vertices. The vertex locations from each subject are projected onto the surface normal of the average shape in MIN152 space. The projection values representing the perpendicular distance from the average surface were stored in a 4D file and group analysis was performed using randomize (FSL, Oxford, UK). Meanwhile, FA maps from all subjects’ DTI data were aligned into MNI152 space using nonlinear registration. The mean FA image was then created and thinned to create a mean FA skeleton. Each subject’s aligned FA data was then projected onto this skeleton, and the resulting 4D FA skeleton data was fed into voxel-wise cross-subject statistics. A randomize procedure (FSL) was used to perform the group analysis (500 permutations). A restrictive statistical threshold was used (cluster-based threshold p < .05, corrected for multiple comparisons).Results and Discussion
As anticipated, we found changes of thalamic morphometry in FM compared to CLBP (Figure 1). The vertex analysis showed significant group differences in right thalamic shape. Compared to the CLBP group, the FM group was associated with significant surface depression in right thalamus (p < .05). Oppositely, the left thalamus did not show any significant morphometric differences between FM and CLBP groups. TBSS analysis showed significantly reduced FA in several white matter tracts of individuals with FM compared to CLBP, including right superior longitudinal fasciculus (slf), right posterior thalamic radiation (ptr), right sagittal stratum (ss), and bilateral corpus callosum (cc) (Figure 2). This finding suggests that patients with FM had distinct abnormalities (in locations and/or degree) in thalamic morphometry and WM microstructural integrity compared with patients with CLBP. Although the precise mechanisms underlying these changes remains unclear, observed reduction of GM and WM integrity may represent a progressive degenerative pain processing, which might reflect the long-term experience of widespread deep pain in FM. Except for the interhemispheric corpus callosum, our results showed an abnormal pattern of laterality in both GM and WM changes. Additional research examining the relationship between these white matter abnormalities and subcortical changes is needed.Acknowledgements
No acknowledgement found.References
[1] Hoy D et al, Ann Rheu Dis. 2014; 73:968-74. [2] Melzack R et al, J Dent Rduc. 2001; 65:1378-82. [3] Apkarian AV et al, J Neurosci. 2004; 24:10410-15. [4] Balike MN et al, PLoS One. 2011; 6:e26010. [5] Buckalew N et al, Pain med. 2008; 9:240-48. [6] Buckalew N et al, Pain Med. 2010; 11:1183-97. [7] Ceko M et al, Hum Brain Mapp. 2015; 36:2075-92. [8] Wolfe F et al, Arthritis Rheum. 1990; 33:160-72. [9] Schmidt-Wilcke T et al, Pain. 2007; 132 Suppl 1:S109-16. [10] Andersson JL et al, Exp Brain Res. 1997; 117(2):192–99. [11] Royce GJ et al, J Comp Neurol. 1985; 235(3):277–300. [12] Patenaude B et al, Neuroimage. 2011; 56(3):907-22.Figures
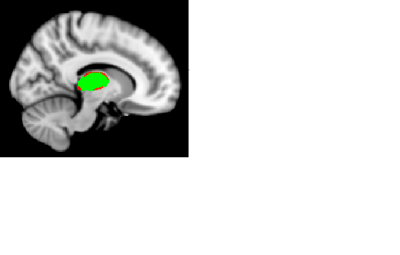
FSL-FIRST vertex analysis showed significant
surface depression in dorsal and ventral aspects of the right thalamus in fibromyalgia
compared to chronic low back pain group. Green color represents right thalamus
mask. Red color represents significant surface depression at p < .05
(corrected for multiple comparisons at cluster level). Thalamus structure is
overlaid on MNI152 T1-weighted brain image.
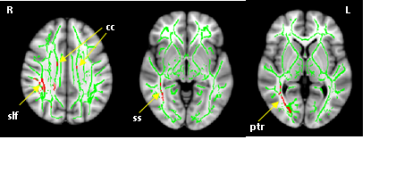
TBSS revealed regions of reduced FA in patients with fibromyalgia
compared to chronic low back pain. Red color represents significant voxels at p
< .05 (corrected for multiple comparisons at cluster level). Mean FA
skeleton of all subjects is overlaid in green on MNI152 T1-weighted brain
image.