3733
MRI Connectivity Impairment Associated with Timing of Seizure Recurrence After Surgery in Temporal Lobe Epilepsy1Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 2Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 3Neurosurgery, Vanderbilt University Medical Center, Nashville, TN, United States, 4Electrical Engineering and Computer Science, Vanderbilt University, Nashville, TN, United States, 5Neurology, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Surgical resection of the seizure focus in the mesial temporal lobe is a common treatment of drug-resistant temporal lobe epilepsy (TLE) with an approximately 80% success rate. Our previous work showed that presurgical MRI-based functional and structural network connectivity can identify those TLE patients with the most unfavorable seizure outcomes. The goal of this work was to increase specificity of our prediction by characterizing those with seizure free and favorable outcomes. The results suggest that when impairment in functional connectivity of the seizure propagation network extends to the contralateral hemisphere, patients will experience rare post-surgical seizures sooner.
Purpose
Surgical resection of the seizure focus in the mesial temporal lobe is a common treatment of drug-resistant temporal lobe epilepsy (TLE). Currently, approximately 80% of these patients become seizure free after surgery1. The ability to identify the remaining 20% of patients would reduce the numbers of failed surgeries, and may motivate more pre-surgical evaluation of these patients so the appropriate treatment can be utilized. In previous work we identified a potential MRI connectivity network model of mesial TLE and demonstrated that similarity to this model may predict favorable post-surgical outcome2. Here we extend this work to examine network differences between those patients with seizure free and favorable outcome to increase specificity of the prediction.Methods
We enrolled 31 patients with temporal lobe epilepsy (16 F, 22 Right TLE, 37.8 +/- 12.1 years) who were scheduled to undergo mesial temporal lobe resection and 44 age and gender matched healthy controls (21 F, 39.3 +/- 14.3 years). Presurgical 3T MRI were acquired on each subject using a 32 channel head coil. The scans included: 1) 3D T1-weighted gradient echo image (repetition time = 9.1 ms, echo time = 4.6 ms, 192 shots, flip angle = 8 degrees, matrix = 256 x 256, 1 x 1 x 1 mm3); 2) T2*-weighted fMRI Blood Oxygenation Level Dependent image at rest with eyes closed for functional connectivity (FC) (matrix = 80 x 80, field of view = 240 mm, 34 axial slices, echo time = 35 ms, repetition time = 2 sec, slice thickness = 3.5 mm/ 0.5 mm gap, 2 x 300 volumes, 2 x 10 minutes); and 3) diffusion weighted MRI for structural connectivity (SC) (b = 1600 s/mm2, 92 directions, 2.5 x. 2.5 x 2.5 mm3). Post-surgical seizure assessments were made using the Engel scoring3 each year after surgery (up to 3 years where available): I-a = seizure free; I-b = auras only; II = rare disabling seizures, almost seizure free, III = worthwhile improvement; IV = no worthwhile improvement. We consider Engel I-II and III-IV as favorable and unfavorable, respectively.
The seizure propagation network included three regions each ipsilateral and contralateral to the seizure onset (hippocampus, insula, thalamus), with two midline bilateral structures (precuneus and mid cingulate). FC was calculated as the correlation between the pairwise regional functional MRI time series. For SC, FSL4 probabilistic fiber tracking was performed using the diffusion MRI, and values of tracks between each seed and target were normalized to region size. Using the control population and their associated ages, patient FC and SC values were transformed to standard deviations from fitted age matched control (FCcorr, SCcorr).
Relationships between the connectivity (FCcorr, SCcorr) and 1 year outcome of those patients with Engel I-II scores were identified (n=21) using Spearman correlation. In addition, a 3 year outcome score was computed as the number of years of Engel I-a or I-b outcome post-surgery (0 to 3 years). Only those with 3 years outcome and without 1 year outcome of Engel III-IV were included (n=17). This score was compared with connectivity using Spearman correlation to identify associations.
Results and Discussion
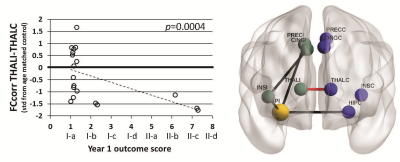
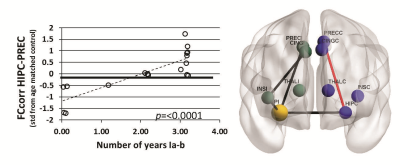
FCcorr between the ipsilateral and contralateral thalamus was negatively correlated with 1 year outcome (p=0.0004) (Figure 1), but few patients had Engel II (rare seizures) outcome at 1 year. FCcorr between the contralateral hippocampus and the precuneus was positively correlated with number of years of Engel I-a (seizure free) or I-b (auras only) outcome after surgery (p<0.0001) (Figure 2). No SCcorr values were correlated with outcome in those with Engel I-II outcome.Conclusions
Our previous work showed that presurgical MRI-based functional and structural network connectivity similarity can separate those TLE patients with the most unfavorable seizure outcomes (Engel III-IV) from those who will get some benefit from surgical resection of seizure focus (Engel I-II). Here we investigated characteristics of the latter subset of patients with Engel I-II 1 year outcomes. Ipsilateral FC including the hippocampus to precuneus is reduced in these patients (black paths in Figures, results not discussed here). Results suggest that when contralateral hippocampal to precuneus functional connectivity is also decreased below healthy controls of the same age, patients will experience rare post-surgical seizures sooner (up to 3 years). If validated, it may be possible to use these methods to predict surgical outcome in individual patients that are not distinguishable with other clinical assessments.Acknowledgements
This work was supported by NIH R01 NS75270 (Morgan).References
1. Englot, D J and E F Chang. Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurgical Review 2014; 37(3): 389-405.
2. Morgan, V L, D J Englot, B P Rogers, B A Landman, A Cakir, B Abou-Khalil and A W Anderson. Magnetic resonance imaging connectivity for the prediction of seizure outcome in temporal lobe epilepsy. Epilepsia 2017; 58(7):1251-1260.
3. Engel, J, P C Van Ness, T B Rasmussen and L M Ojemann, Outcome with respect to epileptic seizures, in Surgical Treatment of the Epilepsies, J. Engel, Editor. 1993, Raven Press: New York. p. 609-621.
4. Jenkinson, M, C F Beckmann, T E Behrens, M W Woolrich and S M Smith. FSL. Neuroimage 2012; 62(2): 782-790.
Figures