3732
Delivery of mHTT to the rhesus macaque brain leads to a reduction in caudate volume in a new monkey model of Huntington’s disease1Neuroscience, Oregon National Primate Research Center, Beaverton, OR, United States, 2Advanced Imaging Research Center, Oregon Health and Science University, Portland, OR, United States, 3Behavioral Neuroscience, Oregon Health and Science University, Portland, OR, United States
Synopsis
Huntington’s disease (HD) is a genetic, neurodegenerative disorder caused by CAG repeat expansion in mutant huntingtin gene (mHTT) on Chromosome 4 and is characterized by degeneration of several brain regions, with the caudate nucleus and the putamen being the most heavily affected. Structural MRI was used to investigate the longitudinal striatal atrophy in a new rhesus macaque HD model, especially the reduction of rostral caudate volume. According to the longitudinal volumetric analysis, the delivery of 82Q mHTT to rhesus macaque striatum leads to a significant reduction in caudate volume. However, no such significant differences following delivery of 16Q mHTT model was observed. These data indicate that viral delivery of 82Q mHTT serves as a nonhuman primate model of Huntinton’s disease that reproduces neuropathological characteristics of the disease.
Introduction Huntington’s disease (HD) is a genetic, neurodegenerative disorder caused by a CAG repeat expansion in mutant huntingtin gene (mHTT) on Chromosome 4. HD is characterized by degeneration of several brain regions, with the caudate nucleus and the putamen being the most heavily affected. Accordingly, patients suffer from motor symptoms, cognitive decline and complex neuropsychiatric alterations. A non-human primate model of HD is valuable to characterize the robust extent of neuropathology and behavioral phenotypes seen in humans, as well as for developing biomarkers of disease progression and assessing potential therapeutics. In this study, structural MRI was used to investigate longitudinal atrophy of the caudate in a new rhesus macaque HD model.
Methods 8 female rhesus macaques were divided into two groups. One group (n=4) was injected bilaterally with an adeno-associated virus (AAV2/1) encoding pathogenic mHTT with 82 CAG repeats (82Q) into the head of the caudate nucleus and the putamen. Another group (n=4) was injected bilaterally with the same virus encoding a control mHTT with 16 CAG repeats (16Q). All animals were scanned with a Siemens Trio 3T MRI system (Erlangen, Germany) at three times; prior surgery (baseline), and 5 months and 10 months after surgery. A 3D T1-weighted MP-RAGE pulse sequence was used to acquire structural volumetric images. A customized surface receive coil was used for the baseline scan. A circularly-polarized transmit, 15-element receive “extremity” coil was used for the other two time points. All MR images were normalized to the INIA19 template coordinate1 using a rigid-body registration method2. This normalization method keeps the brain volume the same as in the original images. Manual segmentation of the rostral caudate was performed on normalized coronal images. Volumetric measurements were taken starting from the rostral-most point of the caudate and ending in the plane intersecting the middle of the anterior commissure. This sub-region of the caudate was selected for imaging analysis because post-mortem histological analyses showed that this area of the caudate was the most heavily transduced with the virus.
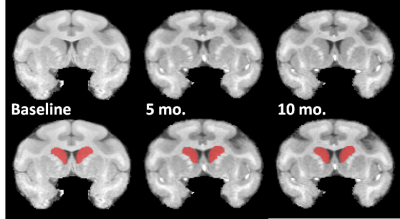
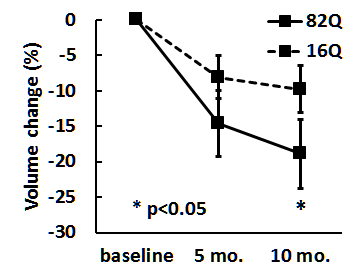
Results Fig. 1 shows normalized MR brain images from an AAV2/1-HTT82Q-injected subject from baseline, 5 month and 10 month time points, as well as manual segmentations of the caudate nucleus. The volumes were obtained for each subject at all three time points. The mean rostral caudate volume changes in each group, expressed as a percent of the volume at baseline, are shown in Fig. 2. Repeated measures ANOVA reveals an effect of time on the volume of rostral caudate. Post-hoc analyses indicate this effect is driven by a significant smaller caudate ROI at 10 months compared to the baseline (p<0.05), in the 82Q animals. Post-hoc analyses did not indicate significant differences between baseline and the 5 month time point in 82Q animals, or between any time points in 16Q animals.
Discussion and conclusion According to the longitudinal volumetric analysis, delivery of AAV2/1-HTT 82Q mHTT to rhesus macaque striatum leads to a significant reduction in caudate volume. However, no such significant differences following delivery of the control AAV2/1-HTT16Q construct was observed. These data indicate that viral delivery of AAV2/1-HTT82Q serves as a valid non-human primate model of HD that reproduces key neuropathological characteristics of the disease seen in human HD patients, including caudate atrophy. Volumetric analysis of the putamen is ongoing.
Acknowledgements
This study was supported by NIH grants P51 OD011092 and R01 NS099136.References
1. Rohlfing T, Kroenke CD, Sullivan EV, et al. The INIA19 Template and NeuroMaps Atlas for Primate Brain Image Parcellation and Spatial Normalization. Front Neuroinform 2012;6:27.
2. Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008;12:26-41.