3730
Longitudinal Diffusion Tensor Imaging in the brain in Friedreich’s Ataxia: follow-up at 12 and 24 months1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
We report 12-month and 24-month longitudinal diffusion tensor imaging (DTI) data in the brain of subjects with Friedreich’s ataxia (FRDA). Significant longitudinal changes were observed in several brain areas (including the corpus callosum, internal capsule and superior corona radiata) in a group of 13 patients over 24 months. Our data suggest that diffusion MRI of the brain could be useful to better understand the impact of FRDA on brain microstructure and connectivity, and to assess the effect of potential treatments on neurodegeneration in upcoming clinical trials in FRDA.
Purpose
Friedreichʼs ataxia (FRDA) is the most common inherited ataxia1. While the spinal cord and cerebellum are the main sites of degeneration, the brain is also affected. A number of cross-sectional brain DTI studies have showed widespread microstructural differences between patients and controls (see2 for review). However, only two longitudinal studies have been reported to date3,4, with somewhat contradictory results. Here we present longitudinal DTI data of the brain in FRDA at baseline and 12- and 24-month follow-up.Methods
Subjects: Twenty-eight subjects with FRDA (age 19.0 ± 7.3 years, 15F, 13M) and 20 healthy age- and gender-matched controls participated in the study. In addition, a subset of patients returned for longitudinal follow-up at 12 months (n=16) and 24 months (n=13). Most patients were at a relatively early stage of the disease (disease duration 5.6 ± 3.8 years, clinical score at baseline 42.7 ± 10.5 out of a maximum of 117 on the validated FARS scale). Datasets from one control and three patients were excluded because of excessive motion artifacts, resulting in n=25 patients and n=19 controls.
DTI: All measurements were performed on Siemens 3T scanners (Siemens, Erlangen, Germany). The standard body coil was used for RF transmission and a 32-channel head coil was used for signal reception. Whole-brain diffusion MRI was acquired with the following parameters: TR/TE = 4246/90 ms; voxel size = 1.8x1.8x1.8 mm3; MB=3; 128 diffusion directions with b-value= 1500 s/mm2 and 15 additional b=0 volumes. Data were acquired in two opposite phase encoding directions (A-P and P-A) and combined to correct for geometric and eddy current distortions5. Data were analyzed with Tract based spatial statistics (TBSS6) using DTI-TK7 for the construction of a study-specific template and nonlinear registration of datasets. Nonparametric permutation inference8 was used to identify differences between controls and FRDA subjects, as well as longitudinal changes in FRDA. White matter (WM) pathways were also automatically delineated by nonlinear registration of the ICBM DTI-81 WM atlas9. These pathways include the medial lemniscus (ML), inferior and superior cerebellar peduncles (ICP, SCP), middle cerebellar peduncle (MCP) and posterior limb of the internal capsule (plIC).
Results
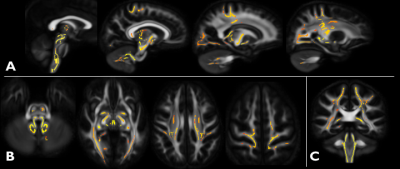
Cross-sectional: Widespread alterations (decrease) of FA in the WM were found (TBSS) throughout the cerebrum, in the cerebellar peduncles (in particular the superior cerebellar peduncle which comprises the cerebellothalamic tract), medulla and upper cervical spinal cord, as shown in Fig. 1. In the cerebrum, areas affected include the WM adjacent to the primary and somatosensory cortices, as well as auditory and visual cortices. The spinocerebellar and corticospinal tracts are also affected. Bilateral differences (p<0.01) were found in all delineated WM pathways, with SCP showing the largest decrease (-24% right and -26% left) in FA and increase (+47% right and +44% left) in radial diffusivity (RD).
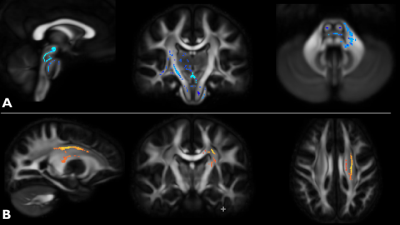
Longitudinal: At 12-month follow-up, we observed an increase in FA with TBSS (paired t-test p<0.05) in the left MCP, SCP, right corticospinal tract and thalamus/thalamic projections (Figure 2A). At 24-month follow-up, we observed a decrease in FA (paired t-test p<0.05) in the left callosal radiations, superior corona radiata and posterior limb of the internal capsule (Fig 2B).
Discussion
The unexpected increase in FA at 12 months (Fig 2A) may reflect compensatory mechanisms occurring in the affected regions. At 24 months (Fig 2B), the observed decrease in FA was consistent with the observed cross-sectional changes and likely reflect neurodegeneration. Rezende et al.4 found similar a similar decrease in FA in SCP, corpus callosum and corticospinal tracts at 12 months in a later-stage cohort (mean FARS score ~ 80).Conclusion
We conclude that significant longitudinal changes in microstructure can be detected in the brain in FRDA over a relatively short period of time using diffusion MRI. This may be useful to better understand disease progression in FRDA and monitor disease progression in clinical trials.Acknowledgements
This work was supported by the Friedreich’s Ataxia Research Alliance, Ataxia UK, GoFAR, the Bob Allison Ataxia Research Center, and NIH grants P41 EB015894 and P30 NS076408.References
1. Pandolfo, M. Friedreich ataxia. Arch Neurol, 2008. 65(10):1296-303.
2. Selvadurai, L.P., et al. Cerebral abnormalities in Friedreich ataxia: A review. Neurosci Biobehav Rev, 2017 (epub ahead of print)
3. Mascalchi, M., et al. Regional Cerebral Disease Progression in Friedreich's Ataxia: A Longitudinal Diffusion Tensor Imaging Study. J Neuroimaging, 2016. 26(2):197-200.
4. Rezende, T.J., et al. Longitudinal magnetic resonance imaging study shows progressive pyramidal and callosal damage in Friedreich's ataxia. Mov Disord, 2016. 31(1):70-8.
5. Andersson, J.L.R., et al. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage, 2016. 125:1063-1078.
6. Smith, S.M., et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 2006. 31(4):1487-505.
7. Zhang, H., et al. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med Image Anal, 2006. 10(5):764-85.
8. Winkler, A.M., et al. Permutation inference for the general linear model. Neuroimage, 2014. 92:381-97
9. Mori, S., et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage, 2008. 40(2):570-582.
Figures