3720
Hyperpolarized [1-13C]pyruvate MRS reveals early-phase transition of energy metabolism in multicellular spheroid tumors1National Institute of Radiological Sciences, QST, Chiba, Japan, 2Incubation Center for Advanced Medical Science, Kyushu University, Fukuoka, Japan, 3Department of Pharmacy, Nagasaki International Unversity, Sasebo, Japan
Synopsis
Hyperpolarized [1-13C]pyruvate MRS was conducted to directly monitor the transition of energy metabolism in tiny multicellular tumor spheroids as a model of early tumorigenesis. As compared with normal tumor cells, the lactate formation from pyruvate was significantly amplified in the tumor spheroids, even in a few hundred micrometer i.d. of smaller ones with no blood vessel formation. These results imply that formation of the sterical structure itself causes the transition of energy metabolism from mitochondria to cytoplasm in tumor tissues. HP [1-13C]pyruvate MRS may thus allow detection of early tumorigenesis by targeting the increased aerobic glycolysis in the initial stage, if much higher performance of HP measurement is realized.
Purpose
Most tumor cells rely on aerobic glycolysis instead of mitochondrial oxidative phosphorylation (OXPHOS) to generate energy (ATP production) for cellular activities1. This distinct glucose metabolism, known as the Warburg effect, is further amplified by an organization of the cells as solid tumor tissues in vivo. However, much has remained elusive; e.g. when and how the metabolic shift is triggered during tumorigenesis. In the present study, hyperpolarized (HP) [1-13C]pyruvate MRS2 was employed to directly monitor the transition of energy metabolism in tiny multicellular tumor spheroids of early-phase3. The HP [1-13C]pyruvate conversion to [1-13C]lactate in the smaller tumor spheroids was noninvasively monitored on an NMR spectra.Methods
Culture of tumor cells and spheroids: All cells were cultured in 10-cm normal culture dishes and maintained in RPMI1640 supplemented with 10% fetal bovine serum and antibiotics. The cells were maintained in a humidified chamber at 37 °C containing 5% CO2. Static spheroidal cultures of tumor cell lines were conducted using 10-cm or 96-well formatted EZSPHERETM (IWAKI) plates. Cells (5 × 104 /ml) were transferred to a 10-cm dish (10 ml) or 96 well (100 μl) and maintained in a humidified chamber at 37 °C containing 5% CO2.
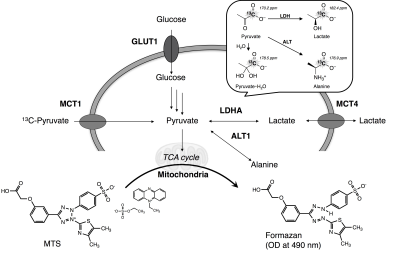
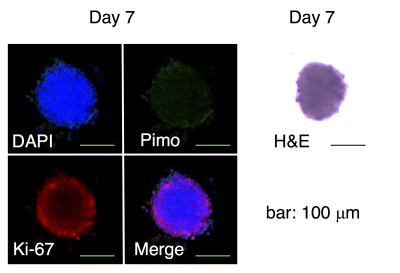
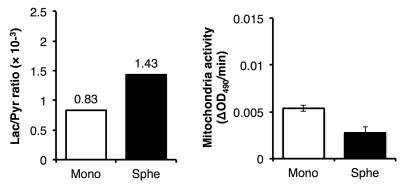
Evaluation of spheroids: Histological analysis was performed to evaluate the cell proliferation (Ki-67), formation of hypoxia (pimonidazole) or necrotic core (H&E). Mitochondrial activity as well as drug resistance were assessed using MTS assay (Fig. 1). The expression level of genes involved in the pyruvate/lactate metabolism was quantified by real-time qPCR4.
HP [1-13C]pyruvate MRS: The HP of [1-13C]pyruvate sample was conducted using HyperSenseTM (3.35 T, 1.4 K, 2.8 mbar, Oxford Instruments, Co. Ltd.). Briefly, a 30 μl aliquot of [1-13C]pyruvic acid (14.2 M) doped with 15 mM OX063 was polarized for 50-60 min by microwave radiation (100 mW) at 93.96 GHz. After polarization, the sample was rapidly dissolved in 4.5 ml of dissolution buffer (100 mM Tris, 100 mg/L EDTA) preheated to 10 bar (~473 K), which warmed the sample to biological temperature (308~313 K, pH 7). The resulting solution was received in a 50-ml reservoir and quickly transferred to a 10-mm NMR tube containing 1-ml suspension of freshly harvested standard culture cells or spheroids. After suspending the samples quickly, the NMR tube was placed in a Japan Redox JXI-400Z spectrometer (9.4 T). Collection of the NMR spectra started 30~35 s after dissolution of HP [1-13C]pyruvate. Tetramethylsilane (TMS, 0 ppm) was used as the external standard for 13C NMR (1-13C in pyruvate: 170.2 ppm, alanine: 176.9 ppm, pyruvate・H2O: 178.5 ppm, and lactate: 182.4 ppm) (Fig. 1). The spectra were recorded using a flip angle of 5° and time of repetition (TR) of 2.5 sec. Resulting 13C NMR spectra were processed using JEOL Delta NMR software v5.0.1 (JEOL Ltd., Tokyo, Japan).
Results and Discussion
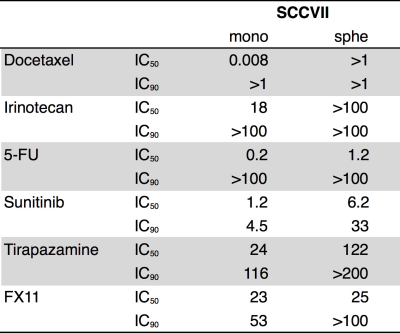
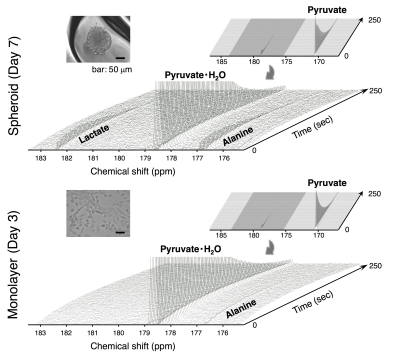
The static culture of murine SCCVII cells by day 7 generated the uniform size (150 μm in median diameter) of cell aggregates that is comprised of actively proliferating cells of the outside layer and quiescent cells of the inside core. No hypoxic and necrotic region observed in this smaller size of spheroids without blood vessel formation (Fig. 2), indicating that the tumor spheroid did not become malignant within this time period. By contrast, this tiny SCCVII cell aggregate conferred resistance to several anti-tumor drugs tested (Fig. 3), possibly due to alteration of the genetic profile involved in the drug actions triggered via formation of the three-dimensional tissue structure. HP [1-13C]pyruvate MRS successfully detected the increase in pyruvate conversion to lactate (1.8-fold) in the spheroids as compared with normal cells (Fig. 4, 5), which was supported by the increase in LDHA and MCT transporters expression on a real-time qPCR. The prostate tumor cells tested in this study5 showed the similar trends as well, highlighting that formation of the sterical structure itself may cause the metabolic shift in tumor tissues, even a few hundred micrometers i.d. of cell aggregates in an initial stage.Conclusion
HP [1-13C]pyruvate MRS revealed a significant increase in lactate formation from pyruvate in tiny tumor spheroids, indicating that tumor cell aggregation itself causes the metabolic shift from mitochondrial OXPHOS to aerobic glycolysis to produce ATP even in the initial stage. Thus, detection of early tumorigenesis might be possible by targeting the transition of energy metabolism with HP[1-13C]pyruvate MRS, if much higher performance of HP measurement is realized.Acknowledgements
We thank to Prof. Shinsuke Sando and Dr. Hiroshi Nonaka (The University of Tokyo) for discussion about this study. This work was supported partly by CREST, the Japan Science and Technology Agency, JSPS KAKENHI Grant Number 17K01363 (Y.T.), 15K21218 (Y.T.), 26560215 (K.I.) and 15H03035 (K.I.). The use of HyperSenseTM was in part supported by the funding program ‘Creation of Innovation Centers for Advanced Interdisciplinary Research Areas’ from JST.References
1. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029-1033
2. Day SE et al., Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med 2007;13(11):1382-1387
3. Tee SS et al., Detection of Tumor Spheroid Metabolism Using Hyperpolarized Magnetic Resonance Spectroscopy. ISMRM 25th Annual Meeting 2017; 5603:
4. Ippolito L et al., Metabolic shift toward oxidative phosphorylation in docetaxel resistant prostate cancer cells. Oncotarget 2016; 7: 61890-61904
5. Scroggins BT et al., Hyperpolarized 13C pyruvate magnetic resonance spectroscopic imaging of prostate cancer tumor xenografts. Cancer Res 2016;76(14 Suppl):Abstract nr LB-184.
Figures