3718
Esterase-catalyzed production of hyperpolarized 13C carbon dioxide in tissues for measuring pHNesmine R Maptue1, Weina Jiang1, Alexander M Funk1, Wei Chen1, Craig R Malloy1,2,3,4, A. Dean Sherry1,2,5, and Chalermchai Khemtong1,2
1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Department of Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, United States, 4Veteran Affairs North Texas Health Care System, Dallas, TX, United States, 5Department of Chemistry, University of Texas at Dallas, Richardson, TX, United States
Synopsis
13C-MRI HP 13C-bicarbonate (H13CO3-) and carbon dioxide (13CO2) is a novel technique for tissue pH mapping. Here, we report 13C-enriched ethyl acetyl carbonate (13C-EAC) for esterase-catalyzed production of HP-13CO2 and HP-H13CO3- for pH measurements. Our results showed that 13C-EAC was rapidly hydrolyzed by esterase to 13C-monoacetyl carbonate, which decomposed to HP-13CO2. Equilibrium between the newly produced 13CO2 and H13CO3- was established and the 13C-NMR signals can be quantified for pH measurements. Finally, in vivo pH measurements using H-13C-EAC was demonstrated in rat livers. These results suggest that HP-13C-EAC is a novel imaging probe for in vivo pH measurements of tissues.
Introduction
The ratio of CO2 to HCO3- has been used as an index of tissue pH using hyperpolarized (HP) 13C-MRI (1). Various salts of 13C-enriched bicarbonate (H13CO3-) and CO2-precurosrs can be polarized using DNP and upon dissolution, the resulting HP-H13CO3- rapidly equilibrates to form HP-13CO2 (2-4). The short T1 and low polarization levels of 13C-bicarbonate salts remain major challenges for this method. In this study, we report the development of HP 13C-enriched mixed anhydrides as esterase-sensitive precursors (Fig. 1) for the HP 13CO2 and H13CO3- buffer pair for tissue pH measurement. We hypothesized that the ester functional group of mixed anhydrides can be readily hydrolyzed by enzyme esterase, producing HP-H13CO3-/13CO2 that can be imaged for estimating tissue pH.Methods
13C-enriched ethyl acetyl carbonate (13C-EAC) was synthesized from 13C-enrihced ethyl chloroformate and acetic acid and isolated as a clear liquid. 13C-EAC was polarized neat for 2 h in a HyperSense polarized using BDPA (40 mM) as a radical. For T1 and signal enhancement measurements, dissolution of HP 13C-EAC was done using ethanol ([EAC] = 2 mM) and 13C NMR spectra were acquired at 9.4 T. For hydrolysis studies of HP 13C-EAC, 2 mL of HP 13C-EAC in PBS was mixed with 2 mL of rat plasma or rat liver homogenate ([13C-EAC] = 2 mM, 37 °C). Series of 13C NMR spectra were acquired with a delay time of 2 s with 10-deg pulses. In vivo pH measurements of rat livers were done in a 4.7T small animal scanner. Healthy Sprague-Dawley rats (250-350 g) were injected with HP 13C-EAC via a tail vein catheter (3 mL, 80 mM). 13C NMR spectra were acquired with 10-deg pulses every 3 s using a volume coil. pH values were calculated from H13CO3-/13CO2 intensity ratios.Results and Discussions
A polarization buildup of 13C-EAC is shown in Figure 2A. 13C-EAC polarization reached the maximum level in a very short time (~30 min). T1 of 13C-EAC measured at 9.4T was 30 s. The liquid state signal enhancement of this HP probe was ~35,000-fold, corresponding to ~28% polarization. Some degree of HP-13C-EAC hydrolysis was observed in PBS as confirmed by the presence of 13C-enriched monoacetyl carbonate (13C-MAC) at 159.4 ppm (Fig. 3). 13CO2 and H13CO3- resonances are also visible at 124.5 and 160.0 ppm, respectively. 13C NMR spectra of HP 13C-EAC in plasma and liver homogenate show strong peaks of 13C-MAC, 13CO2, and H13CO3-. As expected, signal intensities of all 13C-EAC hydrolyzed products are much more pronounced in these biological samples. The presence of enzyme esterase caused rapid hydrolysis of HP-13C-EAC to HP 13C-MAC. The signal-intensity plot of (Fig. 3B) confirms that HP-13C-MAC was produced instantly in plasma and liver homogenate. A HP-13C spectrum of the liver summed over 21-s period after HP 13C-EAC injection is shown in Figure 4A. Our results showed that HP 13C-MAC, 13CO2, and H13CO3- peaks appeared soon after the injection of HP 13C-EAC. HP 13C-EAC signal was very small compared to its downstream metabolites, indicating that 13C-EAC hydrolysis by esterase is very rapid in vivo. Although the HP 13C-MAC and HP H13CO3- peaks in this in vivo result are somewhat overlapped, the two resonances are resolved. Intensities of H13CO3- and 13CO2 in the liver quantified by Gaussian peak fittings are plotted as a function of time in Figure 4B. A ratio between HP-H13CO3- and HP-13CO2 signal intensities was calculated over 21 s (7 time points) while HP 13CO2 signal intensity can be reliably measured. From these values, the average HP-H13CO3--13CO2 signal intensity ratio was 11.9:1, agreeing well with the reported value (5). pH value of the liver was estimated from the calculated intensity ratios (1). The average pH value over this period from 7 values was 7.24 ± 0.08. Interestingly, this value agrees very well with the intracellular pH measured by 31P NMR (6, 7).Conclusions
In conclusion, we have demonstrated that tissue pH can be measured in vivo using HP 13C-EAC. Taking advantage of the abundant and fast mammalian enzymes esterase and carbonic anhydrase, HP 13CO2 and H13CO3- can be rapidly produced in situ and tissue pH can be calculated from HP 13C signal ratios of the physiological buffer pair. The long T1, good chemical stability, and high polarization level of HP 13C-EAC are also advantageous for HP 13C imaging applications, potentially allowing for pre-injection quality control procedures such as radical removal without a significant signal loss. The results suggest that HP 13C-EAC is an attractive pH imaging agent for in vivo assessment of abnormal tissue pH associated with many diseases.Acknowledgements
We thank the following agencies for financial support: NIH 2P41-EB015908 (CRM), NIH 5R37-HL034557 (ADS), and W81XWH-12-1-0134 (CK).References
- Gallagher FA, Kettunen MI, Day SE, Hu DE, Ardenkjaer-Larsen JH, et al. 2008. Nature 453: 940-3
- Gallagher FA, Kettunen MI, Brindle KM. 2011. NMR Biomed 24: 1006-15
- Ghosh RK, Kadlecek SJ, Pourfathi M, Rizi RR. 2015. Magn Reson Med 74: 1406-13
- Korenchan DE, Flavell RR, Baligand C, Sriram R, Neumann K, et al. 2016. Chem Commun 52: 3030-3
- Kerber RC. 2003. Journal of Chemical Education 80: 1437-8
- Cunningham CC, Malloy CR, Radda GK. 1986. Biochim Biophys Acta 885: 12-22
- Malloy CR, Cunningham CC, Radda GK. 1986. Biochim Biophys Acta 885: 1-11
Figures
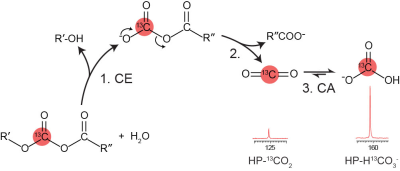
Proposed mechanism of esterase-catalyzed production of
HP 13CO2 and H13CO3-
from 13C-enriched mixed anhydrides. CE = carboxyl esterase; CA =
carbonic anhydrase

(A) A DNP polarization
buildup of neat 13C-EAC polarized with BDPA (40 mM) in a HyperSense
polarizer; (B) Arrayed 13C NMR spectra of HP 13C-EAC (2
mM in ethanol) following ~2 h of polarization acquired every 5 s with a 10-deg
flip angle; and (C) A single 13C NMR spectrum of HP 13C-EAC
showing the resonance of 13C-enriched carbonate carbon at 149.4 ppm

(A) 13C NMR spectra of HP 13C-EAC
(2 mM) in PBS (pH = 7.4), rat plasma, and rat liver homogenate acquired 20 s
after mixing; (B) HP 13C NMR signal-time curves of 13C-MAC,
13CO2, and H13CO3-
normalized to the total HP 13C signal of HP 13C-EAC in
PBS, plasma, and liver homogenate.
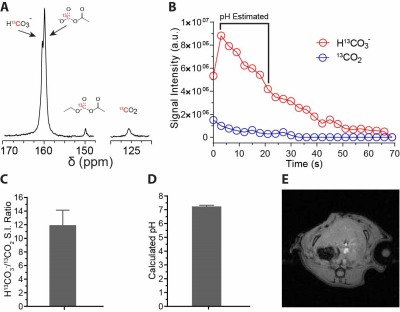
In vivo pH measurements of
a rat liver. (A) A 13C NMR spectrum of rat liver following HP 13C-EAC
administration. The spectrum is a sum of 7 spectra acquired very 3 s over 21 s with
10-deg pulses at 4.7 T; (B) Signal intensities of HP H13CO3-
and 13CO2 from rat liver; (C) Average HP H13CO3-/13CO2
signal ratio calculated over 21 s; (D) Average liver pH value estimated
from the HP H13CO3-/13CO2
ratio; and (E) 1H reference image showing the slice location for 13C NMR.