3717
Developing a therapeutic strategy for a glycolytic cancer model by monitoring on-target in vivo efficacy of a newly developed LDH inhibitor using hyperpolarized 13C Magnetic Resonance Imaging1NCI/NIH, Bethesda, MD, United States, 2National Center for Advancing Translational Sciences (NCATS), NIH, Rockville, MD, United States, 3University of Alabama School of Medicine, Birmingham, AL, United States
Synopsis
This study aimed to develop a new therapeutic strategy with a novel Lactate Dehydrogenase A inhibitor (LDHi) for cancers bearing the Warburg phenotype by monitoring the impact on in vivo metabolic flux of the LDHi using hyperpolarized 13C-MRI. 13C-MRI with hyperpolarized [1-13C]pyruvate revealed in vivo pharmacodynamics and an effective dose of the LDHi without the need for tissuse sampling. In addition, based on these results, we developed a therapeutic strategy with the LDHi for mice harboring a MiaPaCa-2 (a glycolytic pancreatic cancer cell line) xenograft. This methodology can be a novel approach to treat glycolytic cancers.
[Purpose]
Increased lactate production is a characteristic metabolic perturbation in many types of cancer (1, 2), and elevated lactate production is a consequence of increased conversion from pyruvate by Lactate Dehydrogenase A (LDHA). Therefore, LDHA inhibition is a promising new therapeutic approach to treat cancers bearing the Warburg phenotype. To develop a therapeutic strategy, a feasible and sensitive non-invasive imaging approach that can dynamically evaluate the drug’s in vivo on-target efficacy would be highly beneficial since in vitro metabolic profile dose not always predict in vivo cancer metabolism(3).
Hyperpolarized 13C Magnetic Resonance Imaging (MRI) is a valuable technology to investigate metabolic processes in tumor xenografts, allowing us to perform dynamic 13C-metabolic flux analysis in vivo. Use of [1-13C]pyruvate with 13C-MRI provides the ability to monitor LDHA activity through dynamic observation of conversion of [1-13C]pyruvate to [1-13C]lactate. Notably, this technique is safe, repeatable and non-invasive for patients, leading to reducing burden in patients.
Herein, we demonstrate application of 13C-MRI to developing a therapeutic strategy with a newly developed LDH inhibitor (LDHi) for a glycolytic xenograft tumor model.
[Methods]
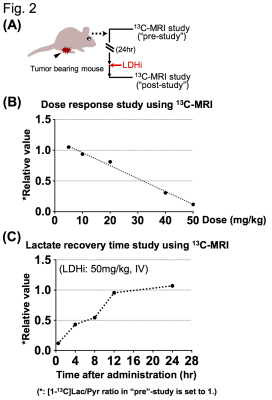
LDH inhibitor: The LDH inhibitor was obtained from NCATS by agreement with National Cancer Institute (NCI) Experimental Therapeutics Program (NExT). Native Gel assay: Native gel assay was performed as described previously(4). After electrophoresis, the gel was soaked in the LDHi for 30 min and then incubated with reaction buffer at 42C for 7-14 minutes. Ex vivo measurement of LDH activity: Xenograft tumors were excised 1 hr after intravenous injection of 50mg/kg-LDHi, and intra-tumoral LDH activity was measured. Hyperpolarized 13C-MRI study: Hyperpolarized 13C-MRI study was performed as described previously(5). [1-13C]pyruvate (30 μL), containing 15 mmol/L OX063 and 2.5 mmol/L gadolinium chelate, was hyperpolarized using the Hypersense DNP polarizer. The hyperpolarized sample was rapidly dissolved in 4.5 mL of a superheated alkaline buffer, and dissolved solution was intravenously injected (12 μL/g body weight). Hyper polarized 13C-MRI studies were performed on a 3T scanner using a 17 mm home-built 13C solenoid coil placed inside of a saddle coil for 1H. 13C-MR spectra were acquired every second with a spectral width of 3330 Hz, repetition time of 1000 ms and flip angle of 10°. 13C two-dimensional spectroscopic images were acquired 27 s after the start of the pyruvate injection, with a 28 × 28 mm2 field of view in a 8 mm axial slice, a matrix size of 14 × 14. Initial 13C-MRI evaluation was performed in a mouse harboring a MiaPaca tumor, 1 day before LDHi injection (“pre-study”), and again after LDHi injection (“post-study”). The LDHi was intravenously injected.
[Results]
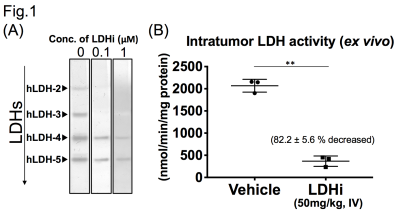
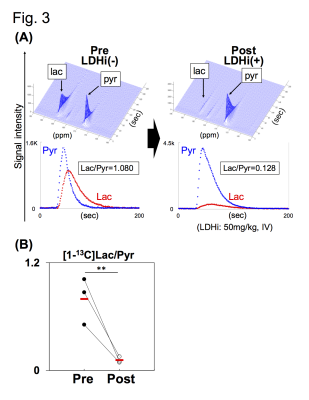
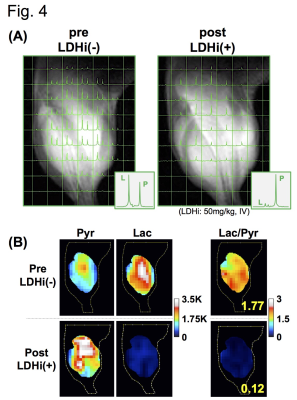
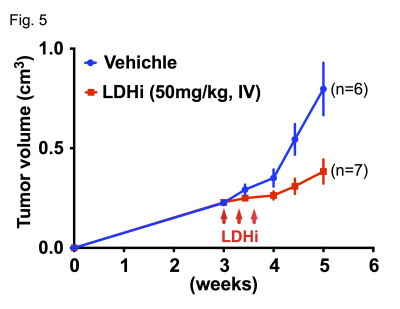
Native gel assay showed that the LDHi dose-dependently suppressed in-gel redox activity of human LDH isoforms (Fig. 1A). In ex vivo assay, LDH activity was significantly suppressed (82.2 ± 5.6 % decreased) in MiaPaCa-2 xenograft tumors by IV injection of 50mg/kg-LDHi (Fig. 1B). Hyperpolarized MR study with [1-13C]pyruvate was performed before and after the LDHi administration to assess inhibitor impact on metabolic flux in vivo. A schematic representation of hyperpolarized [1-13C]pyruvate MR study is shown in figure 2A. 13C-MR spectroscopy revealed that the LDHi suppressed LDHA activity in the MiaPaCa tumors dose-dependently (maximum effective dose: 50mg/kg) and time-dependently (Fig. 2B, 2C). In addition, Lactate production in the tumor was obviously suppressed 30 minutes after intravenous administration of 50mg/kg-LDHi (Fig. 3A), and [1-13C]lactate to [1-13C]pyruvate ratio ([1-13C]Lac/Pyr) significantly decreased (83.3 ± 4.4 % decreased) (Fig.3B). Interestingly, this result correlates closely with the resuts in figure 1B, suggesting that 13C-MRI can monitor in vivo on-target effect of this LDHi without the need for tissue sampling. Chemical Shift Imaging with 13C-MRI also showed that [1-13C]lactate signal in each voxel significantly decreased in the tumor region upon LDHi administration (Fig. 4A). The sum of [1-13C]lactate signals in the tumor region significantly decreased after the LDHi administration, and [1-13C]Lac/Pyr ratio calculated from chemical shift images in total tumor region also significantly decreased from 1.77 to 0.12 (Fig. 4B). Furthermore, intravenous administration of 50mg/kg-LDHi significantly suppressed tumor growth in MiaPaCa-2 xenograft tumors (Fig. 5A).
[Conclusions]
In the current study, intra-tumoral inhibition of LDHA in vivo upon intravenous administration of the LDHi was readily visualized as markedly reduced conversion of [1-13C]pyruvate to [1-13C]lactate. These findings confirm that hyperpolarized 13C-MRI is a useful technology to evaluate on-target in vivo efficacy of the LDHi without the need for tissue sampling. 13C-MRI technology can be highly beneficial evaluating new therapeutic approaches to treat cancers bearing the Warburg phenotype using the LDHi, leading to reducing burden in patients in clinical application of the LDHi in the future.Acknowledgements
This project was supported in part with funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E, through the NExT Chemical Biology ConsortiumReferences
(1) Warburg O, et al. Ueber den Stoffwechsel der Tumoren. Biochemische Z. 1924, 152:319-344
(2) Warburg O. On the origin of cancer cells. Science, 1956, 123(3191):309-14.
(3) MA Neveu, et al. Multimodality Imaging Identifies Distinct Metabolic Profiles In Vitro and In Vivo, Neoplasia 2016, 18 (12): 742–52)
(4) Ilka W, et al. Native electrophoretic techniques to identify protein-protein interactions. Proteomics, 2009, 9, 5214–23
(5) Saito K, et al. 13C-MR Spectroscopic Imaging with Hyperpolarized [1-13C]pyruvate Detects Early Response to Radiotherapy in SCC Tumors and HT-29 Tumors. Clin Cancer Res, 2015, 21(22):5073-81
Figures