3714
HP [1-13C] Pyruvate-derived metabolic biomarkers are an early predictor of lung rejection in the rat lung transplantation model1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2University of Pennsylvania, Philadelphia, PA, United States
Synopsis
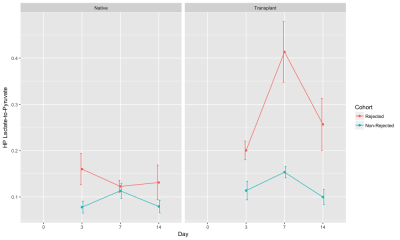
Post-transplant lungs are clinically monitored using regular radiography and/or CT scans to detect rejection. We proposed that a metabolic biomarker may provide higher sensitivity than conventional tools at the onset of rejection, before permanent structural changes in the lungs. Using HP [1-13C] pyruvate CSI, we found that the HP lactate-to-pyruvate ratio in the transplanted lung was significantly elevated in the rejected cohort (~1.7-fold on day 3, ~2.7-fold on day 7) compared to the non-rejected cohort and can be potentionally used as an early predictor of lung rejection.
Introduction
Post-transplant
lungs are clinically monitored using regular radiography and/or CT
scans to detect rejection. We propose that a metabolic biomarker may
provide higher sensitivity than conventional tools at the onset of
rejection, before permanent structural changes in the lungs. Here, we
demonstrated that a hyperpolarized (HP) [1-13C] pyruvate
MRI-based biomarker can predict lung rejection earlier than microCT
in an orthotopic rat lung transplantation model.Methods
Methods: Left lung allografts (rejected) or syngeneic isografts (non-rejected) were transplanted from Fischer to Fischer rats (n= 5, mass= 270±47g), or Wistar Furth to Wistar Furth rats (n= 5, mass= 291±31g), respectively. A triple axis precision system was used to place intrathoracically and stabilize the vascular clips to clamp the bronchovascular structures, thereby avoiding interference with either the heart or contralateral lung movement. A single-suture bronchial anastomosis technique and proximal cuffing approach was used, as previously reported [3]. Animals were monitored post-surgery to assess recovery. Gated micro-computed tomography (μCT) (current= 60mA, voltage= 40kV, reconstructed isotropically at 200 um) was performed on days 0, 3, 7, and 14 to detect structural and ventilation changes. Lungs were segmented using ITK-SNAP to obtain volume and tissue density. HP [1-13C]-pyruvate MR imaging was performed on days 3, 7 and 14. Animals were imaged while supine in a 4.7T magnet (Varian Inc.). HP [1-13C]-pyruvate (28.6mg, 15mM OX063, 1.5mM Dotarem Gd) was polarized using a HyperSense DNP polarizer, and ~1.2mL (4mL/kg, 80mM) of HP agent was injected via the tail vein over 6s. HP [1-13C]-pyruvate chemical shift imaging (CSI) was performed using a 2D slice selective phase-encoded FID-CSI sequence, as previously reported (TR/TE= 35.7/0.35ms, α= 9°, FOV= 45x45x15mm3) [4]. Spectra were reconstructed, processed and analyzed using custom MATLAB scripts.Results
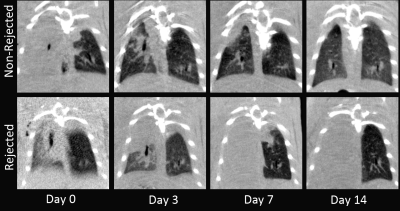
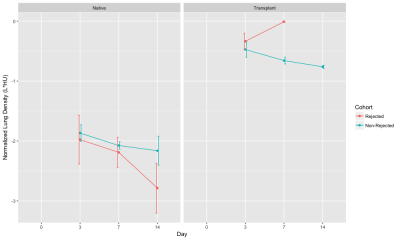
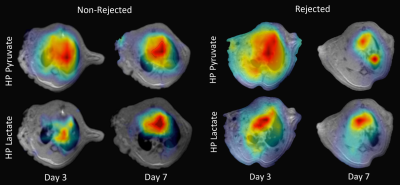
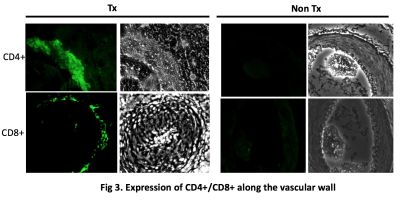
Figure 1 shows representative slices from CT scans on days 0, 3, 7, and 14. By day 3, native (right) lung ventilation in both cohorts was almost fully recovered. By day 7, the non-rejected cohort has complete ventilation, whereas the rejected cohort has no ventilation in the transplanted lung. Figure 2 shows the quantified parameters obtained from the segmented lung CT’s: native lungs in both cohorts are not significantly different on days 3 or 7. Figure 3 shows representative HP pyruvate and HP lactate maps of rejected and non-rejected cohorts on days 3 and 7. Hyperperfusion (via HP pyruvate distribution) is observed on day 3 in both cohorts; by day 7, however, the non-rejected cohort exhibits normal perfusion, whereas the pyruvate is primarily shunted towards the healthy native lung in the rejected cohort. HP lactate is elevated in both lungs on day 3 in the rejected cohort, but remains significantly elevated in the transplanted lung despite minimal perfusion. In comparison, the lactate distribution has returned to baseline in both lungs in the non-rejected cohort, as reflected in their respective lactate-to-pyruvate ratios (figure 4). On day 3, lactate-to-pyruvate is significantly greater in the native lungs of the rejected cohort (0.16±0.08) compared to those of the non-rejected cohort (0.08±0.03) . More importantly, the lactate-to-pyruvate ratio in the rejected transplanted lung (0.20±0.05) is much greater than any other lung, and significantly different (p = 0.013) from the transplanted lung in the non-rejected cohort (0.11±0.04). By day 7, the rejected lung has a lactate-to-pyruvate of 0.41±0.15, which is at least 2.7-fold higher than the next cohort, suggesting full rejection on day 7. Figure 5 shows immunohistochemistry stained slides for T-cell proliferation in both rejected and non-rejected cohorts, suggesting a possible source of the elevated lactate signal.Discussion
CT results show little difference in transplanted lungs between cohorts on day 3, suggesting that in this model CT imaging is unable to predict rejection this early. The elevated pyruvate signal in the native lungs of the rejected and non-rejected transplantations was most likely due to increased perfusion as blood flow was shunted from the recently transplanted left lung to the healthy right lung. Although lactate signal was elevated in the native lung in both cases, the lactate-to-pyruvate ratio was comparable to that of both lungs in the non-rejected cohort. However, both lactate signal and lactate-to-pyruvate ratio in the transplanted lung was significantly elevated in the rejected cohort (~1.5-fold on day 3, ~2.7-fold on day 7) compared to the non-rejected cohort. The source of this increased lactate-to-pyruvate is most likely a combination of hypoxia, inflammation, and early rejection on day 3, whereas it may be primarily driven by the activated CD8+ T-cells by day 7, as suggested by the IHC staining.Conclusions
This study demonstrated that the lactate-to-pyruvate ratio derived from HP [1-13C]-pyruvate MRI can be used as a potential metabolic biomarker to assess the recovery of both native and transplanted lungs post-transplantation.Acknowledgements
No acknowledgement found.References
[1] Naka, et al. cAMP-Mediated Vascular Protection in an Orthotopic Rat Lung Transplant Model. Circulation Research, 1996.
[2] de Perrot, et al. Effect of ventilator-induced lung injury on the development of reperfusion injury in a rat lung transplant model. Cardiothoracic Transplantation, 2002.
[3] Habertheuer, et al. Innovate, simplified orthotopic lung transplantation in rats. Journal of Surgical Research, 2013.
[4] Pourfathi, et al. In-vivo Assessment of Lung Injury Using Hyperpolarized Carbon-13 MRI in a Two-hit Model of Acid Aspiration and VILI. ISMRM, Singapore, 2016.
[5] H. Shaghaghi et al., “Metabolic spectroscopy of inflammation in a bleomycin-induced lung injury model using hyperpolarized 1-13C pyruvate,” NMR Biomed., vol. 27, no. 8, pp. 939–947, Aug. 2014.
Figures