3710
Hyperpolarized [1-13C] pyruvate as a biomarker for treatment monitoring in lymphoma murine models1Department of Physical Therapy and Rehabilitation Science, UCSF, San Francisco, CA, United States, 2Department of Radiology & Biomedical Imaging, UCSF, San Francisco, CA, United States, 3Helen Diller Family Comprehensive Cancer Center, UCSF, San Francisco, CA, United States, 4Oncology Innovative Medicines and Early Development, AstraZeneca, Waltham, MA, United States, 5Oncology Innovative Medicines, AstraZeneca, Cheshire, United Kingdom
Synopsis
In primary CNS lymphoma (PCNSL), MYD88 mutation is the most
Introduction
In primary central nervous system lymphoma (PCNSL), L265P mutation of the myeloid differentiation 88 (MYD88) protein is the most common1. It induces a constitutive activation of IRAK4 kinase, IRAK1 phosphorylation and increased NF-κB signalling, a key player in cell survival, proliferation and metabolism, offering a unique window for therapeutic development. We previously showed that AZ1495, a potent inhibitor of IRAK4, significantly delayed progression and improved overall survival in L265P-mutated PCNSL models2, highlighting the clinical potential of IRAK inhibition. In the clinic, however, early monitoring of such targeted therapies remains challenging using conventional imaging, as treatment often results in non-readily detectable metabolic changes. Because NF-κB controls metabolism as well, we questioned if hyperpolarized 13C magnetic resonance spectroscopic imaging (HP-13C MRSI) could non-invasively monitor IRAK inhibition in PCNSL harbouring MYD88 mutation.Methods
Animals:
Two patient-derived models were studied: one mutant for MYD88 (MYD88mut), and one wild-type (MYD88wt). Mice (n=3 MYD88mut, n=5 MYD88wt) were injected intracranially with 200x103 tumour cells on the left hemisphere. Xenograft bearing animals were imaged around 3 weeks post intracranial injection as the baseline (day 0, pre-treatment). At this point, animals received a daily dose AZ1495 treatment (50mg/kg/day per os) and were imaged at 2 and 4 post start of AZ1495 treatment.
MR acquisitions:
Each imaging session was performed on a 14.1T Varian system using a 1H/13C dual tuned 40mm coil and Hypersense hyperpolarizer. T2-weighted images were acquired using the following parameters: FOV 30mm, TR/TE 1200/20, 1 mm slice thickness. HP-13C MRSI was performed 17sec following injection of 24μL HP [1-13C] pyruvate using a FOV 32mm, TR 67ms, 4mm slice thickness, matrix 16x16.
Data analysis:
For T2-w MRI, signal intensity was measured using ImageJ for the ipsilateral and contralateral sides. Ratios of the ipsilateral over the contralateral intensity was reported to account for possible changes in image gain. Manual segmentation of the whole brain was performed using 3DSlicer. For HP-13C MRSI data, the ratio of HP [1-13C] lactate to pyruvate was measured using SIVIC and MATLAB programs for the lesion site at day 0, 2 and 4.
Results
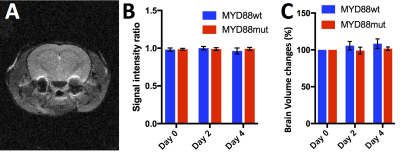
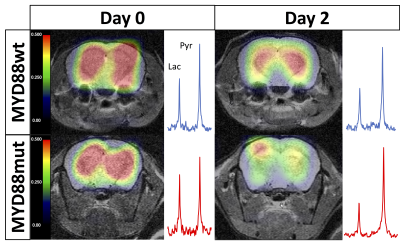
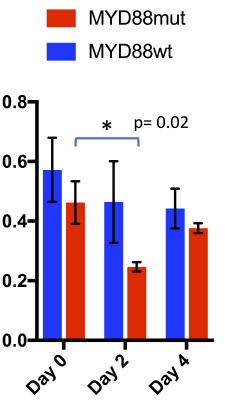
On T2-w MRI (Fig.1A), no significant differences in signal intensity between groups at each time point were observed (p = 0.92, 0.34, 0.30 at days 0, 2 and 4 of treatment), nor between time points within each group (p= 0.79 and p=0.76 for MYD88mut and p= 0.28 and p=0.47 for MYD88wt), at day 2 and 4 of treatment respectively when compared with day 0 (Fig.1B). No changes in brain volumes were observed from baseline at days 2 and 4 (p=0.24 and p=0.32 for MYD88wt and p=0.74 and p=0.32 for MYD88mut respectively) (Fig.1C). In contrast, at day 0 of treatment i.e. 3 weeks post intracranial injection, high production of HP [1-13C] lactate was detected in both MYD88wt and MYD88mut (ratio±SEM 0.41±0.1 MYD88wt; 0.49±0.07 MYD88mut) (Fig.2). No significant changes in HP lactate-to-pyruvate ratios overtime were observed in the wild-type xenograft group at days 2 and 4 (p=0.53 and p=0.41 at days 2 and 4 respectively when compared to baseline). In contrast, in MYD88mut animals, the HP lactate-to-pyruvate ratios dropped significantly by 45% (p=0.02) at day 2 compared to baseline, but not at day 4 (p=0.4), in line with the previously reported effect of AZ1495 treatment in MYD88mut tumours only 2 (Fig.3). No significant changes between MYD88wt and MYD88mut groups were observed at days 0, 2 and 4 of treatment (p= 0.369, 0.151, 0.373 respectively).Conclusion
To conclude, we showed that conventional T2-w MRI cannot readily detect PCNSLs tumours, nor their response to targeted treatment; alternative imaging sequences need to be tested. On the other hand, HP-13C MRSI can detect MYD88-mutation-specific modulations of HP lactate production following IRAK inhibition. Because HP-13C MRSI is clinically translatable and expanding rapidly, this study is of high significance for future clinical trials, to enhance monitoring of PCNSL progression and response to targeted therapies.Acknowledgements
No acknowledgement found.References
1. Choi, J.W., et al., MYD88 expression and L265P mutation in diffuse large B-cell lymphoma. Hum Pathol, 2013. 44(7): p. 1375-81.
2. Geng, H., et al., Targeting NF-KB Activation in Novel Intracranial Models of CNS Lymphoma. Blood, 2016. 128(22): p. 777.
Figures