3708
Evaluating hyperpolarized lactate as a theranostic agent for stroke1School of Health Sciences - Geneva, University of Applied Sciences and Arts Western Switzerland, Geneva, Switzerland, 2Image Guided Intervention Laboratory, Faculty of Medicine, University of Geneva, Geneva, Switzerland, 3Department of Clinical Neurosciences, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, 4Centre d'Imagerie Biomédicale (CIBM), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 5Laboratory of Functional and Metabolic Imaging, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 6Department of Radiology, University of Geneva, Geneva, Switzerland, 7Department of Radiology, University of Lausanne, Lausanne, Switzerland
Synopsis
Stroke is the leading cause of disability and the third leading cause of death worldwide. Lactate administration into the ischemic brain directly after blood reperfusion was found to be neuroprotective. MR with hyperpolarized (HP) probes enables in vivo real-time measurement of biochemical transformations of HP 13C-labeled precursors, including lactate. The aim of this study was to demonstrate the feasibility of probing HP [1-13C]L-lactate metabolism in mice brain after ischemic stroke, and to study the influence of the time window after reperfusion on its conversion in order to evaluate the potential of HP lactate as a theranostic agent for stroke.
INTRODUCTION
Stroke is the leading cause of disability and the third leading cause of death worldwide. 80% of strokes are of the ischemic subtype, which can be treated within a narrow time window of 4.5-6 hr from the ischemic onset by restoring blood flow to the ischemic brain1,2. Consequently, only limited number of stroke patients can receive treatment. Despite promising preclinical results of neuroprotective strategies, there are currently no successful bench-to-bedside translations. It has been shown that lactate administration directly after blood reperfusion protects against ischemia-induced cell death and disability3,4. In a clinical pilot study L-lactate was safely administered to traumatic brain injury patients5. Hyperpolarization techniques boost MR sensitivity and enable in vivo real-time measurement of biochemical transformations of hyperpolarized (HP) 13C-labeled precursors, including lactate6. The aim of this study was to demonstrate the feasibility of measuring HP [1-13C]L-lactate metabolism in mouse brain after ischemic stroke, and to study its conversion to pyruvate at different times after reperfusion in order to evaluate the potential of HP lactate as a theranostic agent for stroke.METHODS
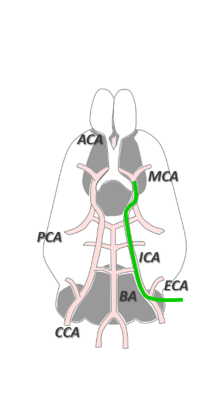
Frozen beads containing sodium [1-13C]L-lactate solution (45-55% (w/w) in H2O) and d8-glycerol (1:1 w:w) with 58 mM TEMPOL radical was dynamically polarized using a custom-designed 7T DNP polarizer (196 GHz/ 1.00±0.05K). C57BL/6J male mice (8 weeks, 25.2 ± 2.4 g) were anesthetized using isoflurane. A 30 min transient middle cerebral artery occlusion (MCAO) was induced as illustrated in Figure 1. Animals were included in the study if the regional cerebral blood flow (rCBF) was reduced to <20% of rCBFinitial during occlusion and increased to >50% of the initial rCBFinitial within 10 min after reperfusion onset. A cannula was inserted into the femoral vein for HP lactate infusion. In order to minimize the exposure to anesthesia, cannulation was performed during the ischemia. Upon reperfusion, animals were placed into a 9.4T/31cm actively shielded animal scanner (Varian/Magnex). MR measurements were performed using a home-built 1H-quadrature/13C-single loop surface coil that was placed on top of the mouse head. B0 inhomogeneity was corrected using FASTMAP protocol. Anatomical T2W images were acquired before infusion of the HP solutions. 325 μL of 90 mM HP [1-13C]L-lactate solution was injected 1 hr or 2 hr post reperfusion (p.r.) in MCAO mice and 1hr post-surgery in sham operated animals. A series of pulse-acquire (30°, BIR-4 pulse) spectra were measured every 3s.RESULTS
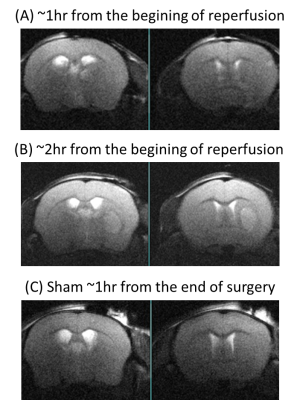
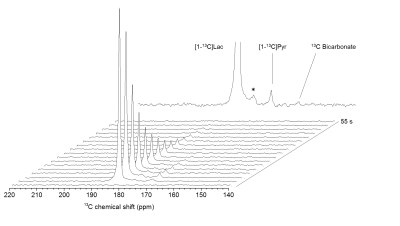
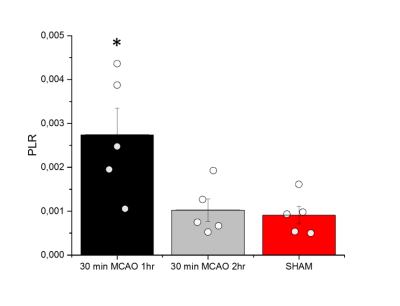
Anatomical T2W images presented in Fig 2 illustrate minor morphological modifications at 1 hr p.r. The characteristic striatal lesion following MCAO is clearly visible at 2 hr p.r. As expected, images of sham animals show no lesion. Infusion of HP [1- 13C]L-lactate lead to the labeling of the pyruvate pool (Fig 3). The time evolution of this conversion was quantified, and demonstrated larger [1-13C]pyruvate signals 1 hr p.r. compared to 2 hr p.r. and sham (Fig 4). Pyruvate-to-lactate ratio (PLR) was then calculated from the summed spectra during the first 37.5 s of the experiment and was corrected to [1-13C]L-lactate concentration in blood at the time of injection for each individual animal (Fig 5). A significantly higher PLR was found 1 hr p.r. compared to 2 hr p.r. and sham (p < 0.05).DISCUSSION
We successfully demonstrated the feasibility to measure the metabolism of a neuro-protective agent, i.e. [1-13C]L-lactate, in mice after ischemic stroke. A significantly higher PLR was found 1 hr p.r. compared to 2 hr p.r. and sham animals, implying a dependency of this ratio on the time elapsed from reperfusion. HP [1-13C]L-lactate crosses the BBB via monocarboxylate transporters (MCTs) and is converted to [1-13C]pyruvate through LDH-mediated exchange. Therefore the labelling of [1-13C]pyruvate is directly related to the endogenous pyruvate pool size7. Thus, the large PLR observed in our study may indicates larger endogenous pyruvate pool. Nonetheless, It has been reported that the BBB plays a limiting role in the observation of cerebral metabolism of HP monocarboxylic-acids8,9. Given that MCT expression and distribution increase after MCAO in mice10, this should result in a larger concentration of HP [1-13C]L-lactate in the brain compartment that rapidly exchange to [1-13C]pyruvate. Further investigations are necessary to clarify the mechanism of this effect in our system. Regardless of the mechanism, it is important to note that higher PLR was measured when no clear morphological changes were depicted in the T2W images, demonstrating the potential of HP [1-13C]lactate as a theranostic contrast agent for molecular imaging after stroke.CONCLUSION
HP [1-13C]L-lactate metabolism can be measured in MCAO mouse stroke model and it is dependent on the time elapsed from reperfusion.Acknowledgements
This work was supported by the Swiss National Science Foundation (grant #310030_170155), the Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, and the Leenards, Jeantet, Biaggi and Juchum Foundations.References
1. Hacke, W. et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England journal of medicine 359, 1317-1329, doi:10.1056/NEJMoa0804656 (2008).
2. Campbell, B. C. et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. The New England journal of medicine 372, 1009-1018, doi:10.1056/NEJMoa1414792 (2015).
3. Berthet, C. et al. Neuroprotective role of lactate after cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 29, 1780-1789, doi:10.1038/jcbfm.2009.97 (2009).
4. Castillo, X. et al. A probable dual mode of action for both L- and D-lactate neuroprotection in cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 35, 1561-1569, doi:10.1038/jcbfm.2015.115 (2015).
5. Ichai, C. et al. Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intensive care medicine 35, 471-479, doi:10.1007/s00134-008-1283-5 (2009).
6. Chen, A. P. et al. Feasibility of using hyperpolarized [1-13C]lactate as a substrate for in vivo metabolic 13C MRSI studies. Magnetic resonance imaging 26, 721-726, doi:10.1016/j.mri.2008.01.002 (2008).
7. Kennedy, B. W., Kettunen, M. I., Hu, D. E. & Brindle, K. M. Probing lactate dehydrogenase activity in tumors by measuring hydrogen/deuterium exchange in hyperpolarized l-[1-(13)C,U-(2)H]lactate. Journal of the American Chemical Society 134, 4969-4977, doi:10.1021/ja300222e (2012).
8. Hurd, R. E. et al. Metabolic imaging in the anesthetized rat brain using hyperpolarized [1-13C] pyruvate and [1-13C] ethyl pyruvate. Magnetic resonance in medicine 63, 1137-1143, doi:10.1002/mrm.22364 (2010).
9. Hurd, R. E. et al. Cerebral dynamics and metabolism of hyperpolarized [1-(13)C]pyruvate using time-resolved MR spectroscopic imaging. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 30, 1734-1741, doi:10.1038/jcbfm.2010.93 (2010).
10. Rosafio, K., Castillo, X., Hirt, L. & Pellerin, L. Cell-specific modulation of monocarboxylate transporter expression contributes to the metabolic reprograming taking place following cerebral ischemia. Neuroscience 317, 108-120, doi:10.1016/j.neuroscience.2015.12.052 (2016).
Figures