3706
Pulmonary Metabolism and Inflammation During Mechanical Ventilation: A Hyperpolarized Carbon-13 Study1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Physiology, University of Pennsylvania, Philadelphia, PA, United States, 3Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
We showed that secondary inflammation due to atelectasis increases pulmonary anaerobic metabolism (lactate-to-pyruvate ratio). Recruitment with PEEP limits anaerobic metabolism and contains injury progression. Measuring tissue metabolism with hyperpolarized MRI can disclose novel pathways of tissue damage during lung injury and assess secondary injury progression in ventilated lungs.
Introduction
Protective ventilation with low tidal volumes (VT) reduces secondary lung injury in patients with acute respiratory distress syndrome (ARDS) (1-4). However, regional stress due to poorly recruited atelectasis may also worsen injury during ventilation (1,4). While the pathophysiology of stretch-induced lung injury has been studied extensively (2), its impact on lung metabolism has not been comprehensively characterized. Increased trans-pulmonary blood lactate is strongly correlated with the severity of lung injury (7), and is associated with increased glycolytic activity in the lungs due neutrophil recruitment and activation (5,6). While FDG-PET has shown increased FDG uptake in regions with inflammatory lesions (8), it does not reveal the fate of 18F-FDG. What is more, PET imaging cannot be used repeatedly to evaluate the lung’s inflammatory status due to long scan times, the long half-life of 18F-FDG, and ionizing radiation. In this study, we used [1-13C] pyruvate MRI in an acid aspiration pneumonitis model to show that alveolar recruitment by positive-end expiratory pressure (PEEP) contains anaerobic metabolism in the lung tissue by limiting inflammatory injury due to atelectasis. Furthermore, our study shows that increased pyruvate-to-lactate conversion in the absence of PEEP is primarily the result of recruitment and activation of neutrophils in the lungs.Materials and Methods
Twenty-one Sprague-Dawley rats (306±10g) were ventilated (VT=8ml/kg, FiO2=1.0, PEEP=5 cmH2O, frequency=52 min-1). Each animal received a hyperpolarized [1-13C] pyruvate injection at healthy baseline, as previously described (9), after which injury was induced in 14 animals via intratracheal instillation of 0.5ml/kg hydrochloric acid (HCl, pH 1.25). Seven rats received 0.5 ml/kg saline in a similar manner (Sham group). A second hyperpolarized [1-13C] pyruvate injection was performed 1 hour after acid/saline instillation. PEEP was then reduced to 0 cmH2O in seven injured rats (ZEEP group) and in the Sham group. Two additional [1-13C] pyruvate injections were performed 2.5 and 4 hours after the acid/saline installation, respectively. Oxygen saturation (SpO2) and pulmonary compliance (Cdyn) were monitored throughout the experiment, after which lungs were fixed, sliced and immunostained with H&E for injury assessment. Additional slices were collected to measure the tissue for expression of intercellular adhesion molecule-1 (ICAM-1) and myeloperoxidase (MPO) using immunofluorescent staining. The ICAM-1 and MPO are markers of neutrophilic binding and activation (10,11), respectively, and were used to further assess the inflammatory status of the lungs.Results and Discussion
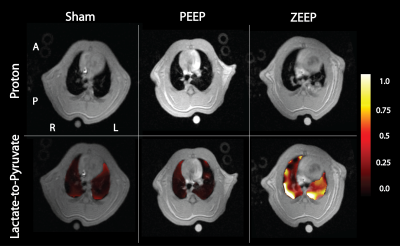
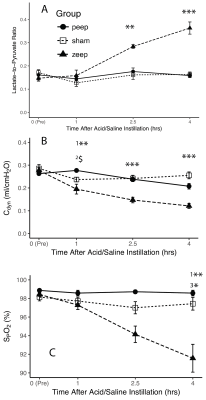
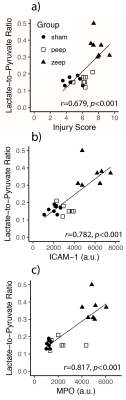
We observed a time-dependent increase of the lactate-to-pyruvate ratio in posterior lung regions in the ZEEP group, which was co-localized with increased proton signal intensity (Figure 1). Although less pronounced, we also observed a gradual increase in the lactate-to-pyruvate ratio map in both the central and anterior lung in ZEEP rats. Lactate-to-pyruvate ratio remained stable over time in both sham and PEEP rats. Average lactate-to-pyruvate ratio was significantly higher in the ZEEP group than in sham and PEEP groups at both 2.5 (p<0.01 for both comparisons) and 4 hours (p<0.001 for both comparisons) after acid/saline instillation (Figure 2.a). Pulmonary compliance (Figure 2.b) declined in all injured rats 1 hour after acid instillation, and continued to decline in the ZEEP group compared to sham and PEEP groups (p<0.001). Oxygen saturation (Figure 2.c) declined over time in the ZEEP group, and was significantly lower than in sham (p=0.003) and PEEP (p=0.035) groups. The decline in the overall lung function assessed by compliance and oxygenation in the ZEEP group confirmed in-vivo progression of lung injury and atelectasis. The H&E stained sections showed that overall lung injury score was strongly correlated with lactate-to-pyruvate ratio (Figure 3.a). Lung injury as assessed by formation of hyaline membranes and edema was significantly higher in the ZEEP group, accounting for the trends of worsening lung mechanics and lower oxygenation. The H&E staining showed widespread alveolar damage and influx of infiltrates into air spaces in both injured groups. We further assessed the inflammatory status of the lungs by immunostaining lung sections followed by fluorescence microscopic imaging to measure the expression of endothelial ICAM-1 and MPO. Both ICAM-1 and MPO were significantly elevated in the ZEEP group and were strongly correlated with the lactate-to-pyruvate ratio (Figure 3.b-c). This trend suggests that enhanced glycolysis and anaerobic metabolism in the absence of PEEP are associated with secondary inflammatory injury to the lungs.Conclusions
In this study, we used hyperpolarized [1-13C] pyruvate MRI to demonstrate that PEEP contains anaerobic metabolism and secondary inflammation in an experimental model of aspiration pneumonitis. In the absence of PEEP, on the other hand, we observed a substantial increase in anaerobic metabolic activity associated with neutrophil recruitment and accumulation. Although preliminary, these findings suggest a new approach to understanding the relationship between lung metabolism, lung damage, and inflammatory cascade during mechanical ventilation.Acknowledgements
References
1. FRANK JA, GUTIERREZ JA, JONES KD, ALLEN L, DOBBS L, Matthay MA. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med 2002;165:242–249. doi: 10.1164/ajrccm.165.2.2108087.
2. Slutsky AS, Ranieri VM. Ventilator-Induced Lung Injury. N Engl J Med 2013;369:2126–2136. doi: 10.1056/NEJMra1208707.
3. Malhotra A. Low-Tidal-Volume Ventilation in the Acute Respiratory Distress Syndrome. N Engl J Med 2007;357:1113–1120. doi: 10.1056/NEJMct074213.
4. Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 1994;149:1327–1334. doi: 10.1164/ajrccm.149.5.8173774.
5. Steinberg KP, Milberg JA, Martin TR, Maunder RJ, Cockrill BA, Hudson LD. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med 1994;150:113–122. doi: 10.1164/ajrccm.150.1.8025736.
6. Miller EJ, Cohen AB, Matthay MA. Increased interleukin-8 concentrations in the pulmonary edema fluid of patients with acute respiratory distress syndrome from sepsis. Critical Care Medicine 1996;24:1448–1454.
7. De Backer D, Creteur J, Zhang H, Norrenberg M, Vincent JL. Lactate production by the lungs in acute lung injury. Am J Respir Crit Care Med 1997;156:1099–1104. doi: 10.1164/ajrccm.156.4.9701048.
8. Bellani G, Guerra L, Musch G, Zanella A, Patroniti N, Mauri T, Messa C, Pesenti A. Lung Regional Metabolic Activity and Gas Volume Changes Induced by Tidal Ventilation in Patients with Acute Lung Injury. Am J Respir Crit Care Med 2011;183:1193–1199. doi: 10.1164/rccm.201008-1318OC.
9. pourfathi M, Xin Y, Kadlecek SJ, et al. In vivo imaging of the progression of acute lung injury using hyperpolarized [1-(13) C] pyruvate. Magn. Reson. Med. 2017;307:2526. doi: 10.1002/mrm.26604.
10. Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J. Appl. Physiol. 1985;59:1978–1985.
11. Jochen Grommes OS. Contribution of Neutrophils to Acute Lung Injury. Molecular Medicine 2011;17:293–307. doi: 10.2119/molmed.2010.00138.
Figures