3705
In Vivo Imaging with Hyperpolarized Aspirin1Cancer Systems Imaging, UT MD Anderson Cancer Center, Houston, TX, United States, 2GI Med Oncology, UT MD Anderson Cancer Center, Houston, TX, United States, 3Clinical Cancer Prevention, UT MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Epidemiological evidence strongly suggests that chronic low-dose aspirin usage leads to a significantly lower incidence of cancer and metastatic disease. Given its emergent role as a chemopreventive, it is highly desirable to develop a method to monitor aspirin pharmacokinetics and metabolism in real time and in vivo. We have recently synthesized and hyperpolarized single and double 13C-labeled aspirin, monitored chemical acetylation in hyperpolarized phantom experiments, and determined metabolism and biodistribution of hyperpolarized 13C aspirin in mice.
Introduction
It is highly desirable to develop a method to monitor aspirin’s pharmacokinetics and metabolism in vivo and in real time. While traditional radiotracer-based methods have been used previously to interrogate the in vivo pharmacokinetics of aspirin, they cannot distinguish between the intact compound and its metabolic by-products. Furthermore, these techniques are not readily adaptable to routine imaging due to cost and safety concerns.
We synthesized and hyperpolarized single and double 13C-labeled aspirin using dynamic nuclear polarization (DNP). Hyperpolarization enables the real-time interrogation of drug metabolism since the polarization is retained on the 13C nuclei in both the injected compound and its metabolites. Metabolic and chemical changes that occur in vivo can be observed as changes in the amplitude and chemical shift of the 13C resonances. In addition, due to the low natural abundance and low gyromagnetic ratio of 13C, there is no 13C background signal. We developed hyperpolarized 13C-labeled aspirin to interrogate chemical transacetylation and general base hydrolysis in phantom experiments and to monitor aspirin metabolism and biodistribution following intravenous injection in mice.
Methods
13C-labeled aspirin derivatives were readily obtained by reaction of either unlabeled or 13C-labled salicylic acid with 1,1-13C acetic anhydride or 1-13C acetyl chloride. For hyperpolarization experiments, aspirin was dissolved in a 63% dimethyl sulfoxide-d6 and water solution, final concentration 1.5 M. Oxo63 free radical (Oxford Instruments) was added directly to the mixture to a final concentration of 15.6 mM. 1.8 mM gadolinium (III) relaxation agent (ProHance) was added into each sample for greater solid-state polarization enhancement. Samples were polarized using a DNP HyperSense polarizer (Oxford Instruments) operated at 100 mW power of 94.09 GHz microwave frequency at a temperature of 1.4 K for 1 to 1.5 hours. Hyperpolarized material was dissolved in 4 ml of 10 mM phosphate buffered deuterium oxide (pH = 7.5) to a final concentration of ~ 42 mM. All phantom experiments were done in a vertical bore 7T Bruker BioSpin NMR with 10 mm broad band probe or 7T MR scanner. Single 13C transients using Waltz decoupling (zgdc pulse) were collected every 6 seconds with a 12° flip angle. For mouse experiments, animals were injected with 200 ml of 42 mM hyperpolarized aspirin via tail vein catheter. All in vivo imaging and spectroscopy was performed with dual tuned 1H/13C volume coil (Doty Scientific) in a 7T Bruker Biospec horizontal bore MR scanner equipped with a single channel for carbon excitation/reception. A series of slice-selective 13C spectra were collected between 1-10 s after injection of hyperpolarized material. The single slice was placed over the majority of the animal and a total of 90 transients (2s time delay between each transient, 15° - 20° gauss excitation pulse, 2048 data points). 13C imaging was performed by converting a 1H Bruker interlaced EPI sequence to 13C (field of view 40x40 mm, 32 mm slice, reference frequency 173 ppm, 30° flip angle, single average taken every second for 12 s).Results
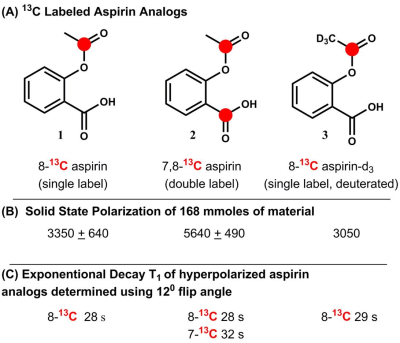
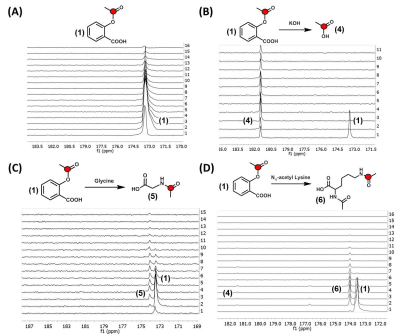
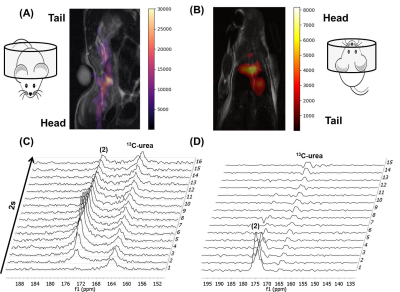
Three different 13C-labeled aspirin analogs were synthesized with >95% purity (Fig. 1A). The chemical shift for the 8-13C-acetyl group of aspirin was observed at 173.4ppm, while the 7-13C-carboxylate carbon from the double labeled analog was observed at 173.5ppm. Solid-phase polarization was reached after 1 to 1.5 hours in the DNP polarizer and ranged from 5640 + 490 (double label) to 3350 + 650 (single label) (Fig. 1B). The observed T1 of the hyperpolarized carbonyl carbon of the acetyl group was consistently found to be between 28 and 29 s for (1) single-, (2) double- and (3) single-labeled and deuterated aspirin (Fig. 1C). Using phantom experiments, we were able to detect hyperpolarized aspirin and its hydrolysis to acetate (4) in 1 M KOH (Fig. 2A/B). Using Schotten-Baumann conditions, we were able to observe chemical acetylation of glycine and Nα-acetyl lysine with hyperpolarized aspirin (Fig. 2C/B). In addition, we were able to observe hyperpolarized aspirin in mice following intravenous injection of 8.4 µmoles of (2) through both imaging (Fig. 3 A/B) and spectroscopy (Fig. 3C/D). Hyperpolarized signal was observed in the inferior vena cava (<5 s post-injection) and the heart (>5 s post-injection). Hyperpolarized (2) was well-tolerated by the mice even after multiple injections over 2 weeks.Discussion
We successfully, synthesized, polarized, and characterized 13C-labeled aspirin derivatives, followed multiple chemical transformations of hyperpolarized aspirin in real-time, and observed hyperpolarized aspirin in mice in both imaging and spectroscopy experiments. We are currently deploying hyperpolarized aspirin to investigate mouse models of inflammation and colorectal cancer.Acknowledgements
We acknowledge grant support from the following: Boone Pickens Distinguished Chair for Early Prevention of Cancer, the Duncan Family Institute, the UT MDACC Colorectal Cancer Moon Shot, MD Anderson Startup Funds, and 1R21CA181994. Dr. Ornelas was supported by a cancer prevention educational award (R25T CA057730, Dr. Shine Chang, PI). We also thank the staff at the MD Anderson Small Animal Imaging Facility (SAIF), particularly Jorge De la Cerda for his assistance with the animal imaging experiments. The Nuclear Magnetic Resonance Facility and Small Animal Imaging Facilty are supported by the MD Anderson Cancer Support Grant CA016672 (DePinho).References
No reference found.Figures