3703
Noninvasive assessment of treatment response to histone deacetylase inhibitor and radiation for pediatric diffuse midline glioma using hyperpolarized carbon-13 metabolic imaging1Radiology, Chonnam National University, Gwangju, Republic of Korea, 2Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 3Neurology, University of California San Francisco, San Francisco, CA, United States, 4Radiation Oncology, University of California San Francisco, San Francisco, CA, United States, 5Neurology, Neurosurgery and Pediatrics, University of California San Francisco, San Francisco, CA, United States
Synopsis
Diffuse midline glioma is one of the most difficult pediatric cancers to treat. This study investigated the feasibility of 13C magnetic resonance metabolic imaging of hyperpolarized [1-13C]pyruvate for monitoring response to novel therapies in diffuse midline glioma. Treatment with panobinostat was associated with a reduction in hyperpolarized lactate and a reduced LDHA activity in an in vitro experiment. Radiotherapy led to a reduction in the ratio of lactate to pyruvate in rats bearing diffuse midline glioma. The results suggest that hyperpolarized 13C metabolic imaging may provide an early noninvasive biomarker to monitor therapy response in diffuse midline glioma.
Introduction
Conventional MR imaging is limited in distinguishing between
true versus pseudo-progression in children with diffuse midline glioma. Hyperpolarized
13C MR metabolic imaging is a promising new technology that provides
a non-invasive imaging approach to monitor changes in functional properties of
tumors. Recent molecular and genetic discoveries from autopsy and biopsy samples
have triggered potential therapeutic strategies such as histone deacetylase
inhibitor (HDACi) panobinostat for treating diffuse midline glioma1,2.
We hypothesize that levels of hyperpolarized lactate, detected by monitoring
hyperpolarized pyruvate metabolism using 13C MR metabolic imaging,
will be modulated in response to treatment with panobinostat and radiation,
which is a clinical standard of treatment, in models of diffuse midline glioma.Methods
In vitro imaging studies were performed with vehicle- and panobinostat-treated (4 nM for 72 hour) H3.3K27M mutant diffuse midline glioma cells (SF8628) using a NMR perfusion bioreactor system3. Normalized lactate was assessed after an injection of 7.5 μL hyperpolarized [1-13C]pyruvate, followed by 13C MR spectroscopy. The activity of lactate dehydrogenase A (LDHA) was compared between control and treated diffuse midline glioma cells using a spectrophotometric assay. For in vivo studies, luciferase modified SF8628 cells were injected directly into the pons of rats. After a single dose 8 Gy radiotherapy (n=3) using a Nucletron microSelectron system (Ir-192), hyperpolarized 13C MR metabolic imaging data were acquired using a clinical 3T scanner with a custom-designed 1H/13C coil. The ratio of lactate to pyruvate (lactate/pyruvate) from the treated animals was assessed and compared to control animals (n=3).Results and Discussion
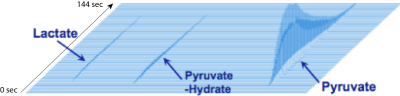
Panobinostat-treated SF8628 cells showed reduced hyperpolarized lactate and LDHA activity: Figure 1 is an example of in vitro hyperpolarized 13C imaging data, showing the delivery of pyruvate and its conversion to lactate. Panobinostat shows significant anti-proliferative effect on SF8628 cells (Fig2A). Pyruvate to lactate conversion was significantly reduced in the panobinostat-treated SF8628 cells compared to control (p=0.03) (Fig 2B). As expected, the reduced lactate signal was associated with decreased levels of LDHA activity compared to control (p=0.03) (Fig 2C).
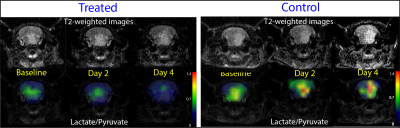
Treatment with radiation led to decrease in lactate/pyruvate compared to control: Rats bearing SF8268 gliomas that were treated with radiation showed a reduction in lactate/pyruvate at day 2 and 4 compared to baseline. In contrast, control rats showed a continuous increase in lactate/pyruvate at day 2 and 4 compared to baseline (Fig 3). Interestingly, both treated and control group showed the increase in tumor volume over the 4-day period, whereas metabolic information obtained from hyperpolarized pyruvate imaging showed a distinct difference in metabolic changes between the treated and control group starting at day 2 from the treatment. This implies that metabolic information obtained from this technique may provide an early imaging marker for therapy response.
Conclusion
The results from this study suggest that hyperpolarized 13C metabolic imaging may be a suitable imaging marker to monitor therapy response in diffuse midline glioma and provide a noninvasive method to predict treatment outcome at an early stage of disease management.Acknowledgements
The support for the research studies came from Kure It Grant for Underfunded Cancer Research, Discovery Grant from American Brain Tumor Association, and the National Research Foundation (NRF) of Korea grant funded by Ministry of Science and ICT (No.2017R1C1B5018396).References
1. Wu G, Broniscer A, McEachron TA et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. Nat Genet. 2012;44(3):251-3.
2. Grasso CS, Tang Y, Truffaux N, et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med. 2015;21(7):827.
3. Keshari KR, Kurhanewicz J, Jeffries RE, Wilson DM, Dewar BJ, Van Criekinge M, Zierhut M, Vigneron DB, Macdonald JM. Hyperpolarized (13)C spectroscopy and an NMR-compatible bioreactor system for the investigation of real-time cellular metabolism. Magn Reson Med. 2010 Feb;63(2):322-9.
Figures