3697
Toward investigating cerebral metabolism using hyperpolarized pyruvate on a human 7T system1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3Philips Healthcare, Dallas, TX, United States, 4Electrical and Computer Engineering, University of Texas at Dallas, Richardson, TX, United States
Synopsis
Advances in hyperpolarized MR technology and pre-clinical investigations have recently led to translational studies using clinical 3T human systems. While hyperpolarization provides large increase in MR sensitivity, spectral dispersion at 3T is limited which makes assessment of various metabolic pathways difficult. This work demonstrates the feasibility of using hyperpolarized 13C-pyruvate to study brain metabolism in a whole-body human 7T system. In particular, the benefit of increased chemical shift dispersion and 1H-decoupling were tested in phantom and rat brains in vivo using hyperpolarized [1-13C]- and [2-13C]-labeled pyruvate. Longitudinal relaxation times of these hyperpolarized substrates at 7T are also reported.
Background
Hyperpolarized MR methods have shown great potential for investigating in vivo metabolism in multiple preclinical disease models, leading to recent translational studies using clinical 3T systems1,2. While hyperpolarized 13C MR spectroscopy provides large sensitivity gains, the limited chemical shift dispersion at 3T can complicate metabolite assignment. For example, assessment of pyruvate carboxylation (PC) using [1-13C]pyruvate is challenging due to insufficient chemical shift dispersion3. This problem could be addressed by utilizing ultra-high field (7T) human systems, and we have recently demonstrated a tracer 13C MRS at 7T that allows for studying metabolic and neurotransmitter biomarkers in human brain4. In addition to PC and glycolysis studies using [1-13C]-labeled pyruvate, [2-13C]pyruvate allows for probing mitochondrial metabolism as the labeled carbon is retained when it enters the TCA cycle. However, this presents several technical challenges5,6, which include large chemical shift displacement artifacts and a 1H-13C coupling in the carbon at position-2 (e.g. [2-13C]lactate and [2-13C]alanine). This report aims to establish a protocol for investigating brain metabolism using hyperpolarized pyruvate on a human 7T system that allows 1H decoupling. Specifically, we measured the T1’s of hyperpolarized [1-13C] and [2-13C]pyruvate, investigated the potential benefits of the large chemical shift dispersion and 1H decoupling in phantom, and performed in vivo rat brain MR spectroscopy with the two substrates.Methods
All 13C spectra were acquired using a whole-body 7T scanner (Achieva, Philips Healthcare) and a human partial volume T/R RF calf coil (RAPID MR International), operating in quadrature for 1H (dual overlapping loop design, loop diameter 12cm) and in linear mode for 13C (loop diameter 6.5cm). Dynamic nuclear polarization of all the 13C-pyruvate samples (35mL of 14M pyruvic acid, 15-mM OX063) was performed for 2-4 hrs using a SPINlab clinical polarizer (GE Healthcare), and the dissolved 70-mM pyruvate solution was hand-carried to the 7T scanner. The distance from the SPINlab to the 7T scanner is ~100m and it was traversed in ~35 sec. For T1 and polarization level measurements, non-localized dynamic 13C spectra were collected from hyperpolarized 13C-pyruvate (FA 5.6o, TR 5s). For in vivo rat brain studies, second-order B0 shimming was applied, and slice-selective dynamic 13C spectra were acquired for 4mins (5.6o Sinc-Gauss RF excitation, slice 10mm, TR 3s, BW 7kHz for [1-13C]pyruvate and 16kHz for [2-13C]pyruvate, spectral points 8192) with a frequency offset centered on the carbonyl region, or on [2-13C]lactate doublet for hyperpolarized [1-13C]pyruvate and [2-13C]pyruvate, respectively. Waltz-16 proton decoupling with 15μT 1H field centered at 4.1ppm (lactate CH) was performed to simplify the spectra and increase SNR. To avoid RF power limitations while still preserving the spectral resolution, the decoupling was turned on only for a duration of the first 20% of the acquisition.Results and Discussion
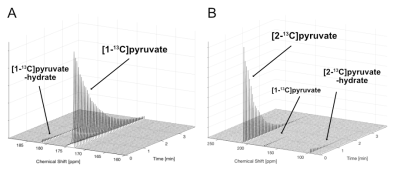
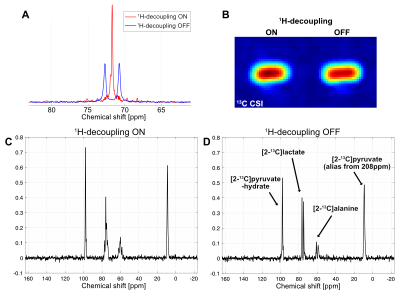
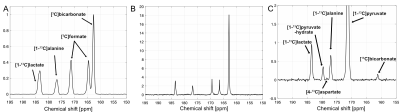
In vitro T1 relaxations of hyperpolarized [1-13C]pyruvate and [2-13C]pyruvate are shown in Fig.1A-B. After correcting for the signal loss from the RF excitations, the longitudinal relaxation times were calculated as 67s and 38s for [1-13C]pyruvate and [2-13C]pyruvate at 7T, respectively, which are 6-s and 12-s shorter than T1’s at 3T (73s for [1-13C]pyruvate and 50s for [2-13C]pyruvate). Due to the long dissolution-to-acquisiiton times (55-65s), the polarization levels were estimated as 7-9% at the start of the data acquisition. 1H-decoupling in a natural abundance lactate phantom increased the peak height of [2-13C]lactate at ~70ppm and its peak-integrated SNR (Fig. 2A-B) by 139% and 6%, respectively. The lactate C2 doublet was not fully decoupled, due to a suboptimal 1H flip-angle calibration and B1 inhomogeneity. Improved decoupling profile and SNR gains are expected in upcoming studies with an optimized transmit gain calibration. In vivo study using hyperpolarized [2-13C]pyruvate with and without 1H decoupling established the feasibility of a protocol for pre-clinical study at 7T (Fig. 2C-D). Furthermore, spectra using hyperpolarized [1-13C]pyruvate display increased chemical shift dispersion of pyruvate and its products in rat brains at 7T (Fig. 3): in addition to lactate, bicarbonate, and alanine, we resolved a peak at 178ppm, which is tentatively assigned to [4-13C]aspartate. This indicates entry of [1-13C]pyruvate into TCA cycle via PC3 in contrast to the 13C-bicarbonate signal that primarily represents the pyruvate conversion via pyruvate dehydrogenase (PDH).Conclusion
In this study, we established the feasibility of performing hyperpolarized pyruvate brain metabolism studies on a commercial high field (7T) human system. Compared to 3T scanners, 7T affords significant gain in spectral dispersion which may allow for assessment of additional metabolic pathways (e.g. pyruvate carboxylation), making it a promising platform for future transitional investigations in metabolism.Acknowledgements
National Institutes of Health of the United States (P41 EB015908, S10 OD018468); The Mobility Foundation; The Texas Institute of Brain Injury and Repair; UT Southwestern Medical Center; Cancer Prevention Research Institute of Texas (RP150456).References
1. Nelson, S. J. et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-¹³C]pyruvate. Sci Transl Med 5, 198ra108 (2013).
2. Cunningham, C. H. et al. Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ. Res. 119, 1177–1182 (2016).
3. Lee, P. et al. In vivo hyperpolarized carbon-13 magnetic resonance spectroscopy reveals increased pyruvate carboxylase flux in an insulin-resistant mouse model. Hepatology 57, 515–524 (2013).
4. Cheshkov, S. et al. Oxidation of [U-(13) C]glucose in the human brain at 7T under steady state conditions. Magn Reson Med (2017). doi:10.1002/mrm.26603
5. Park, J. M. et al. Volumetric spiral chemical shift imaging of hyperpolarized [2-(13) c]pyruvate in a rat c6 glioma model. Magn Reson Med 75, 973–984 (2016).
6. Park, J. M. et al. Measuring mitochondrial metabolism in rat brain in vivo using MR Spectroscopy of hyperpolarized [2-¹³C]pyruvate. NMR Biomed 26, 1197–1203 (2013).
Figures