3696
Fluorine labeling and imaging of biomaterial scaffolds for regenerative medicineMarcin Piejko1,2, Piotr Walczak1,3, Xiaowei Li4, Jeff W.M. Bulte1, and Miroslaw Janowski1,5
1Radiology, Johns Hopkins University, Baltimore, MD, United States, 23rd Department of General Surgery, Jagiellonian University, Cracow, Poland, 3Neurology and Neurosurgery, University of Warmia and Mazury, Olsztyn, Poland, 4Materials Science and Engineering, Johns Hopkins University, Baltimore, MD, United States, 5Mossakowski Medical Research Centre, Warsaw, Poland
Synopsis
There is growing interest in stem cell-based regenerative medicine. Hydrogels can serve as injectable scaffolds for transplanted stem cells in order to provide mechanical support, as well as to mimic natural 3D tissue composition, while allowing for minimally invasive needle- or catheter-based delivery. However, the precision of hydrogel injections, as well as monitoring of gel biodegradation, remains challenging. We have shown that clinical grade fluorine nanoemulsion effectively and firmly labels hyaluronian-based hydrogel, supports survival of embedded stem cells, while only mildly changing rheological properties. Additionally, 19F MRI is very attractive as it does not interfere with anatomical and functional MRI.
Introduction
There is growing interest in stem cell-based regenerative medicine. Hydrogels can serve as injectable scaffolds for transplanted stem cells in order to provide mechanical support, as well as to mimic natural 3D tissue composition, while allowing for minimally invasive needle- or catheter-based delivery. Thiol-modified hyaluronanic acid (HA) is an attractive matrix for cross-linkable hydrogels and has been successfully used in many studies. However, the precision of hydrogel injections, as well as monitoring of gel biodegradation, remains challenging. There are various options for hydrogel labeling including T1 and T2 contrast agents, but in clinical setting their interference with diagnostic MRI might be a major drawback. Therefore, we studied the feasibility of using a fluorine nanoemulsion (V-sense; Celsense) to label hyaluronian-based hydrogels (HyStem, Inc.), as 19F MRI is not affecting anatomical and functional MRI.Methods
Hydrogel labeling: Three hydrogels with various V-sense to hyaluronian volumetric ratios (1:50, 1:10, and 1:5) were prepared, while unlabeled hydrogel served as a control. Biomechanical properties: Gelation time and elasticity were measured by oscillatory stress at 1h and 7 days using an ARES 2 rheometer. Diffusion of fluorine from the hydrogel: 1H and 19F MRI scans were acquired at 1, 3, 7 days, and 2 months after hydrogel preparation using a Bruker Ascend 750 Mhz scanner. 19F MRI included RARE sequence scan with RARE factor=8, FA =90deg, TE/TR: 5.79/1000 ms, NA=32, RFA=180 deg, Nuclei Reference Attenuation: 14dB, Excitation Pulse: length = 0.9133ms, Bandwidth = 3000Hz, scan time: 2 min, 8 sec. The fluorine content was analyzed using Voxel Tracker 2.0. The hydrogels were incubated at 37oC in stationary conditions or agitated at 1000 rpm to additionally force the release of fluorine nanoemulsion from the hydrogels. Cell viability: Luciferase-positive transgenic mouse glial-restricted progenitors (GRPs) were used as prototype stem cells. GRPs were embedded within hydrogels and placed into 96-well plates in triplicate and cell viability/proliferation was measured using bioluminescence (BLI) at 1-, 3-, 7-, 14-, 21-, and 28-day time points. For in vivo experiments, GRPs in V-sense/HA mixtures were injected subcutaneously into SCID mice and BLI was acquired at 1, 3, 7, and 14 days.Results
Increasing concentrations of fluorine gradually elongated the gelation time from 194 s for controls to 304 s for 1/5 V-sense/HA hydrogels (Fig. 1), while their elastic properties slightly decreased, from 183 Pa for the control to 95.3 Pa for 1/5 V-sense/HA hydrogel at day 0, and from 870 Pa for the control to 588 Pa for 1/5 V-sense/HA hydrogel for day 7 (Fig. 2). There was no release of fluorine nanoemulsion from hydrogels maintained in stationary conditions, while there was a decrease in fluorine signal up to 7 days in agitated hydrogels before remaining stable (Fig. 3). No negative influence of V-sense on the proliferation/viability of GRPs was observed either in vitro (Fig. 4) or in vivo (Fig. 5), and, at some time points, the presence of fluorine seemed to have a beneficial effect. Typically, the viability of stem cells decreased over the first 3 weeks, while the rebound and active proliferation was observed at 4 week time point, with higher cellular content in fluorine-labeled hydrogels.Discussion
The formation of the hydrogel was still robust after the addition of a fluorine nanoemulsion, although some changes in the biomechanical properties were encountered. The lack of a significant release of fluorine from the hydrogel over time enables its use for long-term studies, without a negative impact on viability of stem cells. Thus, fluorination of hydrogels appears promising to follow the fate of scaffolded cells non-invasively with MRI.Acknowledgements
Maryland Stem Cell Research Fund: MSCRFI-2829References
No reference found.Figures

Figure 1: Gelation time
measured by oscillatory stress using an ARES 2 rheometer. There was observed
elongation of gelation time from 194 s for control hydrogel to 304s for 1:5
hydrogel.
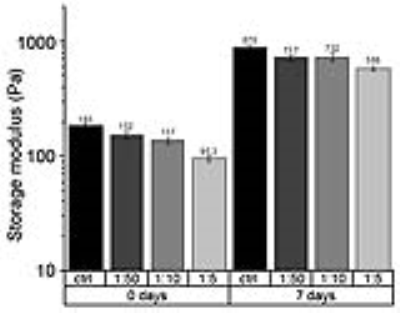
Figure 2: Elasticity
measured by oscillatory stress using an ARES 2 rheometer. The elastic
properties of hydrogel slightly decreased, from 183 Pa for the control to 95.3 Pa for 1/5 V-sense/HA hydrogel
at day 0, and from 870 Pa for the control to 588 Pa for 1/5 V-sense/HA hydrogel at day 7

Figure 3. Magnetic
resonance imaging of 19F labeled hydrogel phantoms. There are
typical cross-sectional (A) and perpendicular (B) images of hydrogel phantoms
and the time course of diffusion of fluorine from hydrogels prepared with
various V-sense (CelSense, Inc.) to HA (Hystem, Inc.) volumetric ratios: 1:5
(C), 1:10 (D) and 1:50 (E). The blue lines represent the hydrogels maintained
in stationary conditions, while the orange lines point on agitated hydrogels.

Figure 4: The time course
of viability/proliferation of GRPs embedded within hydrogels with various
concentrations of fluorine nanoemulsion in comparison to control hydrogel, as
well as to 2D culture of GRPs placed on PLL-coated wells. We see gradual
decrease of signal (number of cells), over the first 21 days, with subsequent
increase of cell number. This scenario corresponds to the transplantation,
where the majority of cells are dying out and the surviving minority provides
the robust cell replacement.
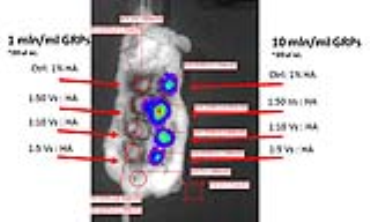
Figure 5: The in vivo imaging of viability of GRPs
embedded within the hydrogels with various concentrations of fluorine, as well
as various cell concentrations.