3695
Small CaF2 nanocrystals as nano-sized tracers for in vivo 19F-MRI1Organic Chemistry, The Weizmann Institute of Science, Rehovot, Israel
Synopsis
In this study we present a novel class of 19F-nanoformulations
based on small (<10 nm) fluoride-nanocrystals (specifically CaF2 nanofluorides)
for MRI applications. We show that homonuclear dipolar interactions can be averaged
out by the fast tumbling of the PEG-coated nanocrystals thus enabling the
acquisition of high-resolution 19F-NMR. Using this feature, we
demonstrate that our newly developed nanofluorides could be used as 19F-MRI
tracers and present a “hot-spot” mapping in an animal model inflammation. The
proposed nanofluorides combine the advantages of using nanocrystals (small,
high 19F-equivalency, maximal 19F-density, and surface
modifiability) with the merits of 19F-MRI tracers.
Introduction
Fluorine-19 MR imaging agents are widely used as tracers that can be directly monitored and presented as a quantitative “hot-spot” maps overlaid on high-resolution anatomical 1H-MRI. Amongst the proposed 19F-traces perfluorocarbon (PFC) nanoemulsions have been successfully used in a wide range of applications1-4 including in clinical setups5. However, the advantages of using nanocrystals based formulations (i.e., controllable composition, crystallinity, size, shape, surface properties, etc.) cannot be applied to PFC-based formulations. In addition, PFCs cannot be obtained as small (<10 nm) nanoparticles and thus cannot be extended for further applications. Here we demonstrate that small (8 nm) water-soluble 19F-nanocrystals (specifically, CaF2 nanofluorides) can average out homonuclear dipolar interactions, enabling to obtain high-resolution 19F-NMR signals of F- in the crystals’ cores. The high 19F-content within the crystalline NPs enables their use as imaging tracers for 19F-MRI and facilitates a “hot-spot” display of their distribution in an animal model of inflammation, enabling in vivo monitoring of inflammatory processes with high specificity in live mice.Methods
Synthesis and characterization: PEGylated CaF2 nanofluorides (CaF2-PEG, CFP, Fig. 1) were synthesized using a solvothermal approach and the purified CFPs were fully characterized (TEM, EDS, XRD, DLS, TGA, and 19F-NMR). The PEGylated NPs were fluorescently labeled by FITC and CY3-based fluorophores by capitalizing on both the -OH and -COOH groups of the PEG coating to allow the validation of their distribution following their injection.
19F-NMR and 19F-MRI: The high-resolution 19F-NMR spectra of nanofluorides were acquired using a 9.4 T NMR spectrometer. The longitudinal (T1) and transverse (T2) relaxation times of the fluoride content in the nanocrystals were estimated prior their use in 19F-MRI studies. Both in vitro and in vivo MRI experiments were performed on a vertical 9.4 T wide-bore MR scanner equipped with double-resonant (1H/19F) 25-mm birdcage RF coil. 1H-MRI: A FLASH sequence with TR/TE=360/4 ms, flip angle=30o, 32 slices of 1 mm thickness, FOV=3.2×3.2 cm2, matrix size=128×128, and NA=2. 19F-MRI: A three-dimensional ultrashort TE (3D-UTE) protocol with a flip angle of 10o, TR/TE=150/0.02 ms, FOV=3.2×3.2×3.2 cm3, matrix size=32×32×32, and NA=8. Mice (N=4) were immunized by subcutaneous injection of 50 µl of immunogenic emulsion into their footpads. Ten days post immunization 1H-MRI and 19F-MRI data sets were collected before and after fluorescently labeled nanofluoride injection (5 mg NPs in 80 µL) subcutaneously into the footpad.
Results and discussion
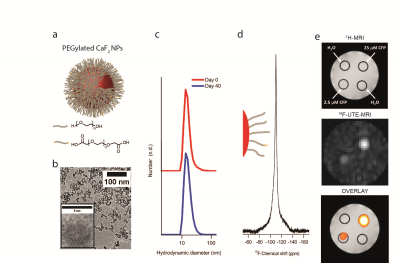
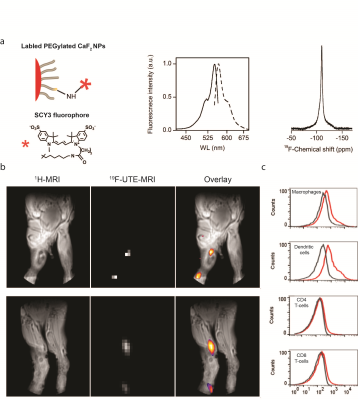
Figure 1 shows that the pure synthesized PEGylated CaF2 ( Fig. 1a) nanofluorides having small size (8 nm core size, Fig. 1b) experience excellent water solubility with small hydrodynamic diameter and overtime stability (Fig. 1c). Figure 1d shows that small water-soluble CaF2 NPs, which tumble fast enough in aqueous solutions, allows the detection of high-resolution 19F-NMR by sufficient averaging of homonuclear dipolar interactions of 19F-nuclear spins within the crystal core. The ability to monitor the small CaF2 NPs with 19F-MRI was demonstrated on a phantom composed of reference samples (no 19F-content) and samples containing two different concentrations of CaF2 NPs (Fig. 1e). By using the ultrashort TE (UTE) sequence that enables MRI of nuclear spin pools having an extremely short T2, a clear 19F-MR signal could be observed from the CaF2 NPs-containing tubes (Fig. 1e, middle) allowing a “hot-spot” representation of their distributions (Fig. 1e, bottom). Surface modification of the PEGylated CaF2 NPs allowed us to produce stable fluorescently labeled derivatives while preserving their high-resolution 19F-NMR properties (Fig. 2a). The potential of using the proposed 19F-nanocrystals as imaging tracers for in vivo 19F-MRI was evaluated in a mouse model of inflammation. Fig. 2b shows a representative in vivo MR imaging of mice with extensive inflammatory activity. The immunized mice were subjected to two 1H- and 19F-MRI sessions that acquired pre- and post- injection of fluorescently labeled CaF2 PEGylated NPs. By using a 19F-UTE-MRI, a clear 19F-signal was observed at the region of the popliteal lymph node (LN) of NP-injected mouse within the same leg of the injection site 1 hour post injection with no 19F-MR signal observed from contralateral regions. Excised cells from the LN of the imaged mice were subjected to FACS analysis that revealed that injected nanofluorides were accumulated mostly in macrophages and dendritic cells (Fig 2c).conclusions
We demonstrated that small fluoride nanocrystals (CaF2) freely tumbling in solution can be studied with high-resolution 19F-NMR and be used as nano-tracers for 19F-MRI. The proposed nanocrysrals elucidate a novel type of 19F-tracers that combine the advantages of using nanocrystals (small, high 19F-equivalency, maximal 19F-density, and surface modifiability) with the merits of 19F-MRI tracers.Acknowledgements
No acknowledgement found.References
1. Ahrens, E.T., Flores, R., Xu, H. & Morel, P.A. In vivo imaging platform for tracking immunotherapeutic cells. Nat Biotechnol 23, 983-987 (2005).
2. Flogel, U. et al. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation 118, 140-148 (2008).
3. Janjic, J.M., Srinivas, M., Kadayakkara, D.K. & Ahrens, E.T. Self-delivering nanoemulsions for dual fluorine-19 MRI and fluorescence detection. J Am Chem Soc 130, 2832-2841 (2008).
4. Ruiz-Cabello, J. et al. In vivo "hot spot" MR imaging of neural stem cells using fluorinated nanoparticles. Magn Reson Med 60, 1506-1511 (2008).
5. Ahrens, E.T., Helfer, B.M., O'Hanlon, C.F. & Schirda, C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med 72, 1696-1701 (2014).
Figures