3694
Gadolinium complexes conjugated with nonsteroidal anti-inflammatory drugs (NSAIDs) as potential inflammation targeting MRI contrast agents.Heekyung Kim1,2, Bokyung Sung3, Soyeon Kim3, Garam Choi3, Ah Rum Baek3, Md Kamrul Islam3, Taekwan Lee4, DongKyu Kim4, Hoe Su Jung4, and Yongmin Chang2,3,5
1Department of Molecular Medicine & BK21 Plus KNU Biomedical Convergence Program, Kyungpook National University, Daegu, Republic of Korea, 2Institute of Biomedical Engineering Research, Kyungpook National University, Daegu, Republic of Korea, 3Department of Medical & Biological Engineering, Kyungpook National University, Daegu, Republic of Korea, 4Laboratory Animal Center, Daegu-Gyeongbuk Medical Innovation Foundation, Daegu, Republic of Korea, 5Department of Radiology, Kyungpook National University, Daegu, Republic of Korea
Synopsis
Inflammation targeting MR CAs conjugated with NSAIDs were prepared according to the general synthetic methods, and characterized by spectroscopic analysis. The relaxivities of theses gadolinium complexes are slightly higher than those of Gadovist®. From in vivo T1-weighted MR images, strong enhancement was observed in the inflammatory region. Also our new gadolinium complexes represent high kinetic inertness in chemically competitive environment compared with other clinically used MR CAs.
Introduction
Inflammation is closely related to cancer progression and several disease.1 NSAIDs (non-steroidal anti-inflammatory drugs) are the most common medications for the treatment of anti-inflammatory actions worldwide. These drugs that bind to inducible enzymes such as COX-1 and COX-2 can be used as inflammatory biomarkers for molecular imaging. Herein GdL1, GdL2 and GdL3 were synthesized from DO3A conjugated to carboxylic acid derivatives of NSAIDs which were diflunisal, fenbufen and sulindac respectively. In this study, relaxivities, kinetic stabilities and animal MR imaging experiments were performed. We expect that these gadolinium complexes conjugated with NSAIDs will potentially be able to target inflammation-induced disease in MRI.Materials and Methods
All reagents were purchased from commercial sources and used as received. The synthesis of all products were confirmed by microanalysis and spectroscopic techniques such as NMR, HR-FAB mass. The kinetic inertness for gadolinium complexes was determined by the evolution of the normalized paramagnetic longitudinal relaxation rates at the transmetallaiton condition.2 T1 measurements were carried out using an inversion recovery method with variable inversion times (TI) at1.5T (64 MHz). For T2 measurements, the CPMG (Carr-Purcell-Meiboon-Gill) pulse sequence was adapted for multiple spin-echo measurements. T1, T2 relaxation times were obtained from the nonlinear least-squares fit of the mean pixel values at variable TI and TE, respectively. The relaxivities (r1 and r2) were then calculated as a slope of linear fit of relaxation rate along with concentrations. The in vivo MR experiments was performed in accordance with the rules of the animal research committee of Kyungpook National University (KNU). For the inflammatory model, 30 µL of turpentine oil was injected into left thigh muscle of ICR mice (27-30 g, six-weed of age, male). Inflammation outbreak was maximized after 3 days of injection. The mice were anesthetized by 1.5% isoflurane in oxygen. MR images were acquired before and after intravascular injection of each MR contrast agents (dose: 0.1 mmol Gd/kg). The images were acquired with a 1.5 T (GE Healthcare, Milwaukee, WI, USA) equipped with a homemade small animal volume coil. The imaging parameters for SE (Spin echo) were as follows: repetition time (TR) = 300 ms; echo time (TE) = 13 ms; 10 mm field of view (FOV); 192×160 matrix size; 1.0 mm slice thickness; number of acquisition (NEX) = 8.Result and Discussion
Scheme 1 shows the synthesis of GdL1, GdL2 and GdL3. Relaxivities of these complexes are slightly higher than those of Gadovist® as shown table 1. The kinetic stability of these gadolinium complexes were determined with time-dependent longitudinal relaxation rate (R1,p(t)/R1,p(0)) and the relaxivity was maintained above 95% against to initial value during the measurement. This kinetic stability was comparable with Dotarem® employing the same type of macrocyclic chelate (Figure 1). In vivo MR experiment, all of these complexes exhibit strong enhancement at liver, urinary bladder and gallbladder as seen in Figure 2. Also inflamed tissue of left thigh exhibits strong enhancement more than 2 hours demonstrating inflammation targeting ability of the present agents. The degree of signal enhancement with GdL1, GdL2 and GdL3, as expressed by contrast-to-noise ratio (CNR) in the inflamed tissue, is even higher and persists longer than Gadovist® confirming inflammation targeting nature of these complexes (Figure 3). Especially, GdL3 shows the most intensive enhancement in the inflamed site for long time compared with GdL1 and GdL2.Conclusion
In conclusion, we have synthesized three kinds of inflammation targeted MR CAs by conjugating NSAIDs to DO3A-based ligand. The kinetic inertness of these complexes compares well with that of structurally related Dotarem®. The Gadolinium complexes are able to target inflamed tissue, as confirmed by MR images of mice with inflammation. As anti-inflammatory activities vary depending on the type of conjugated NSAIDs as well as on the structure of the complexes, more detailed studies on structure-targeting activity relationships are likely to deepen insight into the future direction of inflammation targeting MR agents based on gadolinium complexes.Acknowledgements
No acknowledgement found.References
(1) Arthur Ho-Hon Leung, Jiefu Jin, Shuxia Wang, Hao Lei, and Wing-Tak Wong, Bioconjugate Chem., 2014, 25, 1112-1123
(2) Sophie Laurent, Luce Vander Elst, Frederic Copoix, Robert N. Muller, Invest. Radiol., 2001, 36, 115-122
Figures
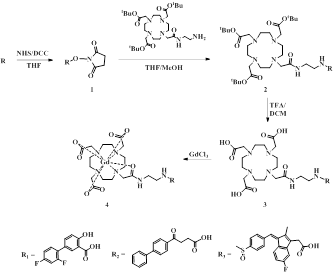
Scheme 1. Synthesis
of GdL1, GdL2 and GdL3.

Table 1. Relaxivities
of GdL1, GdL2, GdL3 and Gadovist®.

Figure 1. Kinetic stability study of inflammation
targeting MR contrast agents and several clinical MR CAs.

Figure 2. In vivo T1-weighted MR coronal
and axial images of inflammatory mice, Pre- and post-injected images of GdL1 (b) GdL2 and (c) GdL3 with 0.1 mmol/kg dose.
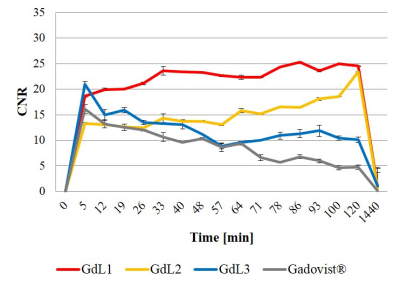
Figure 3. CNR profiles of inflammatory tissue in
respect of GdL1, GdL2, GdL3 and Gadovist®