3693
Manganese Complex of EDTA-Ethoxybenzyl Conjugate as a New Hepatobiliary MRI Contrast Agent1Medical and Biological Engineering, Kyungpook National University, Daegu, Republic of Korea, 2Department of Molecular Medicine & BK21 Plus KNU Biomedical Convergence Program, Kyungpook National University, Daegu, Republic of Korea, 3Institute of Biomedical Engineering Research, Kyungpook National University, Daegu, Republic of Korea, 4Laborstory Animal Center, Daegu Gyeongbuk Medical Innovation Foundation, Daegu, Republic of Korea, 5Department of Radiology, Kyungpook National University, Daegu, Republic of Korea
Synopsis
The purpose of the current study is to design and synthesis of novel Mn2+ complex as an alternative to well-established Gd3+ chelates for use as a liver-targeting MR imaging agent. This new complex exhibits high R1 relaxivity (2.3 mM−1 s−1) than clinically approved Mn-DPDP® (1.6 mM−1 s−1) at 1.5 T. It is also kinetically much inert than that of Magnevist®. In vivo MRI enhancement pattern compares well with those of liver-specific MRI CAs such as Primovist® and Multihance®. It shows greater tumor detection in a liver tumor model with negligible toxicity in a clinical dose.
Introduction
Magnetic resonance imaging (MRI) is a non-invasive powerful diagnostic technique. The contrast of the resulting image often can be enhanced by injection of paramagnetic agents. Though, gadolinium (Gd) is the popular choice amongst the paramagnetic metals due to its suitable magnetic properties. However, Gd based imaging probes are associated to the initiation of nephrogenic systemic fibrosis (NSF).1 In addition, recently a number of reports demonstrated that intravenously administered Gd accumulates in the brains of the patients with normal renal function.2 In this context, progressive approaches have been made based on non-lanthanide metals, particularly less toxic and bio-friendly manganese are receiving special attention. Herein, we report the design of a novel Mn2+ complex based on an ethylenediaminetetraacetic acid (EDTA) coordination cage comprising an ethoxybenzyl (EOB) moiety with high chelation stability for use as a hepatobiliary agent.Materials and Methods
All reagents were purchased from commercial sources and used without further purification unless otherwise stated. Solvents were purified and dried using standard procedures. Deionized water was used for all experiments. T1 measurements were carried out using an inversion recovery method with a variable inversion time (TI) at 1.5 T (64MHz, GE Healthcare, Milwaukee, WI, USA). For T2 measurements, the CPMG (Carr-Purcell-Meiboon-Gill) pulse sequence was adapted for multiple spin-echo measurements. T1 and T2 relaxation times were obtained from the non-linear least squares fit of the mean pixel values for the multiple spin-echo measurements at each TI value and echo time. Relaxivities (R1 and R2) were then calculated as an inverse of relaxation time per mM. MR images of anaesthetized mice were obtained pre- and post-Mn-EDTA-EOB (0.05 mmol Mn/kg) injection by tail vein with a 1.5 T MR unit (GE Healthcare, Milwaukee, WI, USA) using home-made small animal RF coil. The imaging parameters for SE (Spin echo) were as follows: repetition time (TR) = 300 ms; echo time (TE) = 12 ms; 8 mm field of view (FOV); 192×128 matrix size; 1.2 mm slice thickness; number of acquisition (NEX) = 8.Results and Discussion
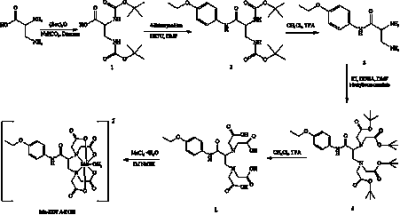
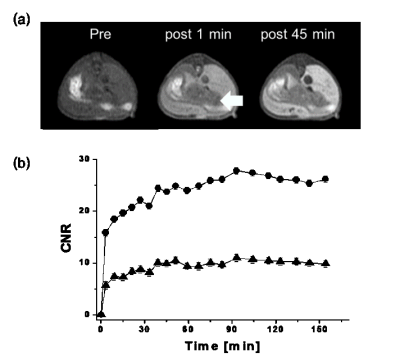
The synthesis method of Mn-EDTA-EOB complex are depicted in Scheme 1. The formation of new compounds was confirmed by microanalysis and spectroscopic methods such as, 1H NMR, HR-FAB-MS. Its R1 relaxivity (6.3 mM-1s-1) in human serum albumin (HSA) solution is higher than MRI CAs such as MnDPDP® (5.2 mM-1s-1) indicating an interaction between HSA and Mn-EDTA-EOB chelate. The kinetic inertness of Mn-EDTA-EOB complex is much higher than that of commercially available MRI contrast agent Gd-DTPA. T1-weighted MR images were obtained by 8-week-old male Institute of Cancer Research (ICR) mice after a bolus injection of Mn-EDTA-EOB through tail vein (Figure 1). It shows strong signal enhancement in liver, kidney, gallbladder and heart. In combination with bio distribution study, in vivo MR images confirm that Mn-EDTA-EOB is excreted through both the liver and kidneys, which is similar to that of the clinically used liver-specific agent Gd-DTPA-EOB and Gd-BOTPA.3 In the hepatobiliary pathway, Mn-EDTA-EOB is taken up by varying degrees of functioning hepatocytes and excreted via the biliary trees, confirming hepatobiliary uptake. Finally, In a liver cancer model, Mn-EDTA-EOB shows significantly improved tumor detection and characterization, suggesting this new complex can be a prominent MR imaging agent for liver cancer (Figure 2).Conclusions
In this study, we successfully synthesized and biological evaluation of Mn-EDTA-EOB chelate as a new family of stable hepatobiliary MRI contrast agent. This new complex can be suitable MR imaging agent for liver cancer.Acknowledgements
No acknowledgement found.References
1. Grobner, T. Gadolinium a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 2006, 21, 1104–1108.
2. Kanal, E.; Tweedle, M. F. Residual or Retained Gadolinium: Practical Implications for Radiologists and Our Patients. Radiology, 2015, 275, 630–634.
3. Hamm, B; Staks, and Lange, L. et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology, 1995,195, 785–792.
Figures