3692
M2 Tumor Associated Macrophage Targeted Magnetic Resonance Imaging Using Mannose Conjugated Anti-Biofouling Iron Oxide Nanoparticle Probes1Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States, 2Radiology, Long Hua Hospital, Shenzheng, China, 3Emory University School of Medicine, Atlanta, GA, United States, 4Surgery, Emory University School of Medicine, Atlanta, GA, United States
Synopsis
By reducing non-specific macrophage uptake, anti-biofouling polymer coated iron oxide nanoparticles (IONPs) conjugated with mannose as the ligand (Man-IONP) was developed for specifically targeting sub-type tumor associated macrophage M2 with mannose receptor 1 expression. This novel MRI probe demonstrated specific M2 macrophage targeting in vitro with M1 and R264.7 macrophages as controls. Immuno-staining of 4T1 breast tumor tissues with Man-IONPs showed the probe targeting M2 similarly to anti-CD206 antibody. NIR and MR imaging of 4T1 tumor-bearing mice showed significant contrast changes after receiving i.v. injection of NIR dye labeled Man-IONP, while little change was observed for mice receiving non-targeted IONP.
Introduction
Microphages play various roles in the immune system and its responses to a broad spectrum of diseases and disorders. The polarization of macrophages leads to two major phenotypes: pro-inflammatory M1 and anti-inflammatory M2. Increasing evidence has shown that tumor associated macrophages (TAMs) contribute to the development and maintenance of tumor microenvironment and tumor-related inflammation,1 with M1 found to be inhibitory to tumor growth while M2 to be responsible for stimulating angiogenesis, suppressing adaptive immunity, and promoting cancer growth and metastasis.2 Since TAM polarization towards the immuno-suppressive phenotype (i.e., M2) is a hallmark of most cancers, specific MRI of M2 TAMs in tumor potentially provides a non-invasive approach for tumor development monitor and imaging-guided drug delivery. However, imaging or targeted delivering drugs to a specific subtype of macrophages with nanoparticles is challenging due to strong non-specific phagocytosis of exogenous materials by all types of macrophages. Here, we reported an anti-biofouling poly ethylene glycol-b-poly allyl glycidyl ether copolymer coated IONPs to develop subtype-specific MRI probe for imaging and image-guided delivery to M2 TAMs using mannose receptor 1 (CD206) as target while eliminating non-specific uptake of the probe by others.MATERIALS AND METHODS
Preparation of Man-IONPs: Anti-biofouling IONPs showing no uptake by macrophages are prepared as previous described.3 Mannose is carboxyl-functionalized by reacting with chloroacetic acid and then conjugated to amines on IONPs via EDC/NHS coupling method.
Macrophages polarization: M1 and M2 macrophages are induced by lipopolysaccharide (LPS) and interleukin 4 (IL-4), respectively, according to literature.2
Immuno-staining of M2 containing tumor tissue: 4T1 breast cancer tumor tissues are used for ex vivo testing M2 targeted Man-IONPs. Anti-CD68, anti-CD163, and anti-CD206 antibodies are used in combination for identification of M2 TAMs. Immuno-staining was performed following manufacture protocol. Man-IONP and anti-CD206 antibody are mixed before applying to the tumor tissue for comparison of CD206 targeting.
Orthotopic 4T1 tumor model: The breast cancer model was prepared by directly injecting 5×106 4T1 cells subcutaneously into the two mammary glands of 7-week female Balb/C mice bilaterally. In 3 weeks, orthotopically xenografted breast tumors typically reached 1 cm in diameter and were ready for use.
Magnetic resonance imaging (MRI) experiment: Tumor-bearing mice were scanned using a 3-Tesla scanner with a small 12-channel surface coil before and after injected with targeted or non-targeted probes (5 mg Fe/kg body weight) labeled with near infrared dye NIR830 via tail vein at 30 hours. The accumulations of probes were validated by in vivo and ex vivo optical imaging using IVIS® imaging system before and after MRI scan. MRI contrast and signal changes were evaluated using T2-weighted fast spin echo sequence and multi-TE spin echo sequence for T2 mapping. The image analysis was done using the region of interest (ROI) metho.
RESULTS AND DISCUSSION
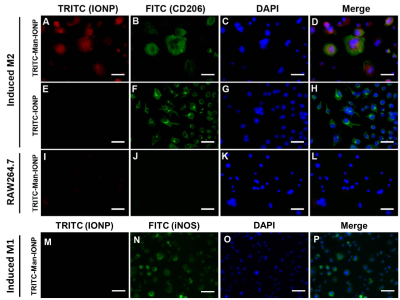
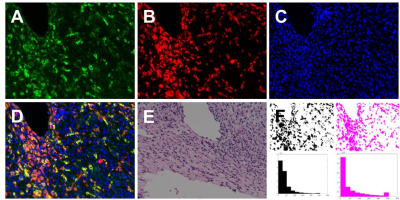
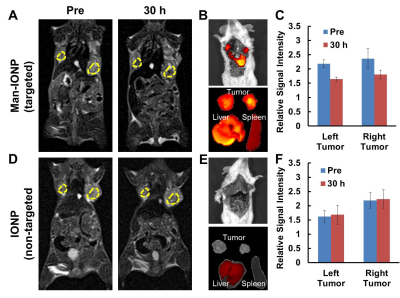
The coincident TRITC and FITC signals for M2 cells treated with Man-IONP indicated the IONP-internalized cells to be M2 (Fig 1A to D), with little uptake was observed for non-targeted IONP (Fig 1E to H). Raw264.7 (Fig 1I to L) and M1 macrophages (Fig 1M to P) were used as control for Man-IONP to confirm of its targeting specificity to M2 macrophages. Man-IONP and anti-CD206 antibody exhibited highly co-localized distribution in immuno-staining of 4T1 tumor tissue (Fig 2A, B, and F), suggesting Man-IONP is as specific and effective as anti-CD206 antibody on targeting CD206 of M2 TAMs. As the confirmation of M2 TAMs requires immuno-staining using multiple antibodies, the combination of anti-CD68, anti-CD163, and anti-CD206 antibodies were used to identify M2 TAMs. Man-IONP is only compared with anti-CD206 antibody because both target the same receptor. MRI of 4T1-tumor-bearing mice at 30 hours after injecting M2-targeted Man-IONP showed ~25% signal intensity drop within tumors comparing with the pre-contrast imaging (Fig 3A and C). Mice receiving non-targeted IONPs did not exhibit measurable signal change 30 hours after injection (Fig 3D and F). To confirm MRI findings, ex vivo optical imaging of the mice were performed to visualize the accumulation of NIR830-labeled IONP in mice. Tumors from Man-IONP injecting mice showed high fluorescence signal (Fig 3B), while little signal was detected from the mice injected with non-targeted IONP (Fig 3E). M2 TAMs in the tumor tissues were identified by immunohistochemistry using anti-CD68 and anti-CD163 antibodies. Only NIR830-labeled Man-IONP demonstrated highly co-localized distribution with M2 TAMs, indicating the probe induced contrast change in MRI came from the cellular uptake of Man-IONP by M2 TAMs.Conclusion
By reducing non-specific macrophage uptake, reported mannose-conjugated anti-biofouling IONP exhibits targeting capability as specific and sensitive as anti-CD206 antibody to M2 TAMs in 4T1 tumor. Probe-induced MRI contrast change can be observed in tumor-bearing mice. Immunohistochemitry staining confirmed the co-localization of M2 TAMs and Man-IONPs, suggesting contrast change attributes to the presence of M2 TAMs.Acknowledgements
This study is supported by NCI’s Cancer Nanotechnology Platform Project (CNPP) grant (U01CA151810-02) and NIH R01CA154846-02.References
[1] Mantovani A. et al., Nat. Rev. Clin. Oncol. 2017; 14:399-416. [2] Song M. et al., ACS Nano 2016; 10:633-47. [3] Li Y. et al., J. Mater. Chem. B 2015; 3:3591-603.Figures