3687
Quantitative Imaging for Development of PET/MRI phantoms1Radiology and Biomedical Imaging, University of California - San Francisco, San Francisco, CA, United States, 2Radiology, Washington University, St. Louis, MO, United States, 3National Institute of Standards and Technology, Boulder, CO, United States
Synopsis
This project aims to develop PET/MR phantoms to evaluate MR-based attenuation correction (MRAC) methods and allow standardization of PET results across PET/MRI scanners. This will be accomplished using quantitative ultrashort echo-time (UTE) imaging methods for measurement of T1, T2* and proton density of in vivo tissue types and candidate phantom materials, along with CT measurements of attenuation coefficients. UTE methods are required to characterize bone and choose bone mimics, as this is the most challenging tissue type for MRAC methods to capture.
Purpose
PET reconstructions require estimates of the 511 keV photon attenuation due to the subject as well as imaging hardware. Generating attenuation coefficient (AC) maps from CT is relatively straightforward1, as CT provides a direct measure of electron density. However, no MRI parameters provide direct mapping of AC maps. The purpose of this project is to develop a PET/MRI phantom to evaluate MRAC methods and allow standardization of PET results across PET/MRI scanners.
The main challenge in MR-based attenuation correction (MRAC) is to accurately capture bone, which has the highest AC but is typically invisible with conventional MRI2. Current MRAC methods rely on anatomical atlases3 or, more recently, deep learning models that can learn anatomical relationships4–6. In light of these variable methods, an ideal PET/MRI phantom would be made of materials that reflect the T1, T2/T2*, PD, and AC values of various tissue types (e.g. soft-tissue, fatty tissue, and bone), and are anthropomorphic.
Methods
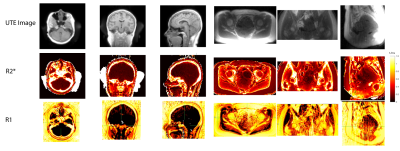
We developed and tested quantitative imaging methods to determine typical T1, T2*, and PD values in vivo and for testing candidate phantom materials. Ultrashort echo time (UTE) pulse sequences were used to capture the rapidly decaying signal from bone. T2* mapping was accomplished by shifting the TE between TRs. T1 mapping was accomplished using a variable flip angle (VFA) approach7,8. All scans used a 3D UTE sequence.
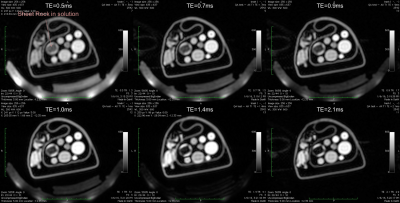
In vivo scans were performed with 2.0 mm (brain) or 2.5mm (pelvis) isotropic resolution. T1 mapping used TR = 11 ms, and 8/44 deg flip angle for VFA T1 mapping. T2* mapping used 6 TEs between 50 µs and 2 ms, with a TR = 6ms. Scan times were approximately 10 minutes.
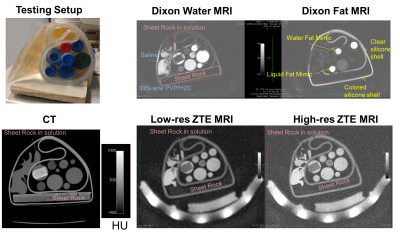
A set of candidate materials including doped water, gel, liquid fat mimic, solid fat mimic, and gypsum (sheetrock, a potential bone mimic) were scanned with quantitative MRI as well as Dixon9 and ZTE-based10,11 MRAC sequences. They were also scanned with CT to provide an estimate of electron density and AC values.
Results & Discussion
In vivo T1 and T2* maps demonstrate the feasibility of our quantitative imaging approach for bone, where bone showed lower T1 and T2* values that is consistent with prior work8,12,13.
The candidate phantom materials results show several interesting findings. First, soft-tissue and fat mimics has similar HU values to tissue, and their MR signal intensities were also similar to tissue with Dixon and ZTE scans that have been used for MRAC. Thus, the fat and soft-tissue mimics available were relatively successful.
For a bone mimic, gypsum (sheet rock) was imaged. On its own, we observed almost no signal from sheet rock. Immersing the sheet rock in water gave MRI appearance that was qualitatively similar to in vivo bone – both show no signal on Dixon MRI scans but some signal on ZTE MRI that is lower than the soft-tissue mimics. The HU value of gypsum in water was also similar to bone of moderate density, with HU values around 500. The T2* was measured to be ~ 0.5-1 ms, which is slightly longer than cortical bone. Modulating the mixture of solution as well as doping may allow for improved correspondence of MRI and AC parameters for a bone mimic using gypsum and bone.
Conclusion
We demonstrated methodology for defining the properties of PET/MRI phantom materials based on in vivo quantitative UTE MRI. We also demonstrated exploration of candidate materials for such a phantom, with promising results for soft-tissue and fat mimics as well as a bone mimic with a gypsum and water mixture. The results of this work will be crucial in designing PET/MRI phantoms to standardize PET results across vendors and institutions.Acknowledgements
NIH grant R01CA212148References
1. Kinahan PE, Townsend DW, Beyer T, Sashin D. Attenuation correction for a combined 3D PET/CT scanner. Med Phys. 1998 Oct 1;25(10):2046–2053.
2. Samarin A, Burger C, Wollenweber SD, Crook DW, Burger IA, Schmid DT, von Schulthess GK, Kuhn FP. PET/MR imaging of bone lesions–implications for PET quantification from imperfect attenuation correction. Eur J Nucl Med Mol Imaging. 2012 Jul;39(7):1154–60. PMID: 22526955
3. Sekine T, ter Voert EE, Warnock G, Buck A, Huellner MW, Veit-Haibach P, Delso G. Clinical evaluation of ZTE attenuation correction for brain FDG-PET/MR imaging—comparison with atlas attenuation correction. J Nucl Med [Internet]. 2016; Available from: http://jnm.snmjournals.org/content/early/2016/06/22/jnumed.116.175398.abstract
4. Han X. MR-based synthetic CT generation using a deep convolutional neural network method. Med Phys. 2017 Apr 1;44(4):1408–1419.
5. Leynes AP, Yang J, Wiesinger F, Kaushik SS, Shanbhag DD, Seo Y, Hope TA, Larson PEZ. Direct PseudoCT Generation for Pelvis PET/MRI Attenuation Correction using Deep Convolutional Neural Networks with Multi-parametric MRI: Zero Echo-time and Dixon Deep pseudoCT (ZeDD-CT). J Nucl Med. 2017 Oct 30;jnumed.117.198051. PMID: 29084824
6. Liu F, Jang H, Kijowski R, Bradshaw T, McMillan AB. Deep Learning MR Imaging–based Attenuation Correction for PET/MR Imaging. Radiology. 2017 Sep 19;170700.
7. Fram EK, Herfkens RJ, Johnson GA, Glover GH, Karis JP, Shimakawa A, Perkins TG, Pelc NJ. Rapid calculation of T1 using variable flip angle gradient refocused imaging. Magn Reson Imaging. 1987;5(3):201–8. PMID: 3626789
8. Han M, Rieke V, Scott SJ, Ozhinsky E, Salgaonkar VA, Jones PD, Larson PEZ, Diederich CJ, Krug R. Quantifying temperature-dependent T1 changes in cortical bone using ultrashort echo-time MRI. Magn Reson Med. 2015 Sep; PMID: 26390357
9. Wollenweber SD, Ambwani S, Lonn AHR, Shanbhag DD, Thiruvenkadam S, Kaushik S, Mullick R, Qian H, Delso G, Wiesinger F. Comparison of 4-Class and Continuous Fat/Water Methods for Whole-Body, MR-Based PET Attenuation Correction. IEEE Trans Nucl Sci. 2013 Oct;60(5):3391–3398.
10. Wiesinger F, Sacolick LI, Menini A, Kaushik SS, Ahn S, Veit-Haibach P, Delso G, Shanbhag DD. Zero TE MR bone imaging in the head. Magn Reson Med. 2015 Jan; PMID: 25639956
11. Leynes AP, Yang J, Shanbhag DD, Kaushik SS, Seo Y, Hope TA, Wiesinger F, Larson PEZ. Hybrid ZTE/Dixon MR-based Attenuation Correction for Quantitative Uptake Estimation of Pelvic Lesions in PET/MRI. Med Phys. 2017.
12. Du J, Bydder GM. Qualitative and quantitative ultrashort-TE MRI of cortical bone. NMR Biomed. 2012 Dec; PMID: 23280581
13. Horch RA, Nyman JS, Gochberg DF, Dortch RD, Does MD. Characterization of 1H NMR signal in human cortical bone for magnetic resonance imaging. Magn Reson Med. 2010 Sep;64(3):680–7. PMID: 20806375
Figures