3684
Metabolic reprogramming in the heart and lung in a murine model of pulmonary arterial hypertensionJose Luis Izquierdo-Garcia1,2, Teresa Arias1, Yeny Rojas1, and Jesus Ruiz-Cabello2,3
1CNIC, Madrid, Spain, 2CIBERES, Madrid, Spain, 3Universidad Complutense de Madrid, Madrid, Spain
Synopsis
Pulmonary Arterial Hypertension is a rare disease of the pulmonary circulation that produces narrowing of small pulmonary arteries, increasing of pulmonary vascular resistance and right ventricular failure. We studied the associated metabolic reprogramming in lung and heart tissues, which is essential for disease progression, in a mouse model of hypoxia induced PAH. Lung and heart metabolism were monitored by HR-MAS NMR spectroscopy and PET imaging. We identified an alteration in energetic and proliferative metabolism of the lungs. We also found a shift in energy metabolism in cardiac tissue.
Background
Pulmonary Arterial Hypertension (PAH) is a rare disease of the pulmonary circulation that produces gradual narrowing of small pulmonary arteries, leading to progressive increase of pulmonary vascular resistance and, ultimately, right ventricular failure and death. Similar to cancer cells, pulmonary endothelial cells of patients with PAH show increased glycolysis and altered glucose oxidation [1]. According to these data, Xu et al. [2] demonstrated that the pulmonary vascular endothelial cells of patients with PAH proliferate and exhibit a phenotype similar to the cancer cells, a shift in glucose metabolism from oxidative phosphorylation to glycolysis.Objective
To test the hypothesis that PAH is characterized by a wider metabolic reprogramming in lung and heart tissues which is essential for disease progression and therefore can be targeted in the treatment of PAH patients. A secondary goal is to identify novel biomarkers for monitoring the evolution of the disease and the normalization of this metabolic reprogramming with potential treatment.Material and Methods
A mouse model of hypoxia induced PAH was investigated. Adult C57BL/6 mice were exposed during 3 weeks to hypoxic conditions, confined in a ventilated chamber under 10% of oxygen and just left the chamber once per week to get subcutaneous injections of the VEGF inhibitor, Semaxanib (SU-5416) (MedChem Express. Stockholm, Sweden). SU-5416 was suspended in CMC (0.5% [w/v] carboxymethylcellulose sodium, 0.9% [w/v] sodium chloride, 0.4% [v/v] polysorbate 80, 0.9% [v/v] benzyl alcohol in deionized water) and injected at 20mg/kg weekly. Normoxic control mice were kept in a regular oxygenated room. In vivo 18F-FDG uptake was analyzed using dual-head PET combined with CT (Bioscan, Mediso). Nuclear imaging was conducted prior to the induction of hypoxic conditions (Basal) and after one, two, and three weeks of hypoxia (n=8) or normoxia (n=8). 25 animals per group were sacrificed at 3 weeks after treatment. Lung and heart tissue were analyzed by HR-MAS 1H NMR spectroscopy on a 500 MHz Bruker spectrometer. Principal Component Analysis was performed using the Metabonomic R package to determine the differences between PAH and control animals. Pulmonary Hypertension was characterized by echocardiography and histology analyses. Echocardiographic studies were conducted before (basal) and three weeks after normoxic/hypoxic conditions. Paraffin-embedded lung tissue sections were stained with Elastic van Gieson to measure the medial thickness of the lung arteries or with pico-Sirius red to measure lung collagen deposition.Results
Metabolomic profiling of lung and RV samples discriminated between NMX and HPX+SU mice (Fig. 1-A). These metabolites point to an alteration in energetic and proliferative metabolism of the lungs. Specifically, we observed in lung tissue a reduction in the concentration of glucose and free fatty acids, and an increase in lactate, alanine, glycine, glutamate, glutamine, taurine, glycerophosphocholine, and phosphocholine in HPX+SU compared to NMX mice (Fig. 1-B). We also found a shift in energy metabolism in cardiac tissue, with higher metabolic concentrations of glutamine, creatine phosphate, lactate, taurine, and glycine in the hypoxic group (Fig. 2). The altered or adapted energy metabolism was confirmed using in vivo PET imaging. Lung 18F-FDG uptake in the lung significantly increased in HPX+SU mice after the first week versus the initial pre-hypoxic exposure conditions and compared to NMX mice (Fig. 3). 18F-FDG uptake was also significantly increased in HPX+SU ventricles versus initial conditions and NMX mice (Fig. 4) after first week of hypoxia exposure.Discussion
This study determined that PAH leads to a wide range of metabolic changes. The pulmonary vasculature in PAH displays a normoxic activation of hypoxia-inducible factor (HIF-1a), which promote a metabolic shift towards aerobic glycolysis (Fig. 5). These significant alterations induce an increase in glucose uptake and a reduction of glucose flux into the mitochondria. As a result, TCA cycle activity is decreased, and the activity of the glutamine pathway that replenish the intermediates of the TCA cycle is increased. Alterations in lipid metabolism were also confirmed by the presence of a reduced concentration of free fatty acids. We have also identified other new biomarkers of cell proliferation in the lung, such as glycine and choline metabolism. In addition, we monitored the specific RV metabolic alterations induced by pulmonary overpressure. We confirmed higher lactate and alanine concentrations and higher FDG uptake. We also identified highly significant biomarkers of RV hypertrophy such as glutamine and taurine. Finally, our study demonstrates that PAH development is characterized by wider metabolic reprogramming in lung and heart tissues and the study of metabolic changes in the VEGF inhibition mouse model of PAH may become a valuable tool for testing new treatments for this severe disease.Acknowledgements
Supported by the Spanish Ministry of Economy, Industry and Competitiveness (MEIC-AEI) grants SAF2016-79593P and SAF2014-59118-JIN, and by European Union's Seventh Framework Programme (FP7/2007-2013) under grant agreement ITN-FP7-608027. J.L.I.G is a CNIC IPP COFUND Fellow and has received funding from the People Programme (Marie Curie Actions) of the FP7/2007-2013 under REA grant agreement nº 600396. T.A is a M+Visión COFUND Advanced Fellow and has received funding from Consejería de Educación, Juventud y Deporte of the Comunidad de Madrid and FP7-PEOPLE-291820 programme. The CNIC is supported by MEIC-AEI and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (MEIC award SEV-2015-0505).References
1- Rehman et al. Adv Exp Med Biol, 661: 171-185.
2- Xu et al. Proc Natl Acad Sci U S A, 104(4): 1342-1347.
Figures
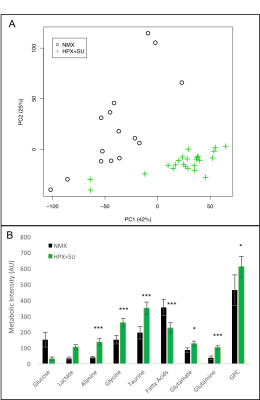
Metabolomic profiling of lung samples from normoxic (NMX, n=15) and SU
5416 hypoxic (HPX+SU, n=22) mice. (A) Principal components analysis (PCA)
performed on 1H-MRS data of lung tissue reveals a clear separation between NMX
and HPX+SU mice. (B) Metabolites were quantified in order to distinguish the
groups. Statistical significance was determined using a Bonferroni-corrected
Student’s t test (* P<0.05; ** P<0.01; *** P<0.001). AU= Arbitrary
Units
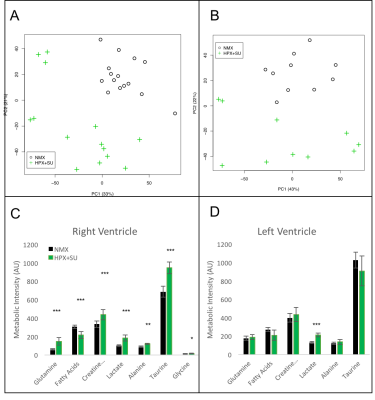
Metabolomic profiling of heart samples from normoxic (NMX) and SU 5416
hypoxic (HPX+SU) mice. (A) Principal components analysis (PCA) performed on
1H-MRS data of right ventricle tissue reveals a clear separation between NMX
(n=15) and HPX+SU (n=14) groups along the first two principal components. (B)
PCA performed on 1H-MRS data of the left ventricle discriminated between NMX
(n=10) and HPX+SU (n=10) groups. Metabolites with the potential to distinguish
the groups were quantified in the right ventricle (C) and left ventricle (D).
Statistical significance was determined using a Bonferroni corrected Student’s t
test (* P<0.05; ** P<0.01; *** p<0.001).
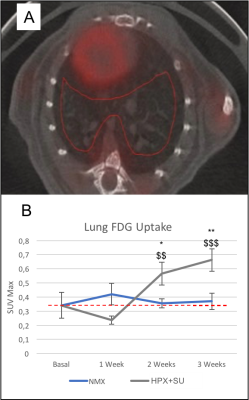
Detection of enhanced aerobic glycolysis in hypoxic SU 5416 mouse
lungs. (A) 3D-ROIs (in red) were drawn on fused PET/CT images for lung
parenchyma 18F-FDG uptake. (B) Increased 18F-FDG uptake was measured at
baseline (time 0) and after one, two, and three weeks of normoxic/hypoxic
conditions. 18F-FDG uptake in SU 5416 hypoxic mice (HPX+SU) significantly
increased in the lung after the first week versus the initial pre-hypoxic
exposure conditions and compared to normoxic mice. The dotted line indicates
basal conditions. * P<0.05; ** P<0.01; *** P<0.001 vs. basal
conditions. $ P<0.05; $$ P<0.01; $$$ P<0.001 vs. NMX group.
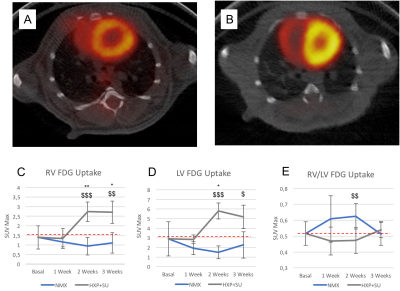
Detection of enhanced aerobic glycolysis in hypoxic SU 5416 mouse
hearts. Representative 18F-FDG positron emission tomography (PET)/computed
tomography (CT) images of normoxic (A) and hypoxic SU 5416 (B) mice at the end
of the three-week protocol. 18F-FDG uptake in SU 5416 hypoxic mice (HPX+SU) was
significantly increased after the first week versus the initial pre-hypoxic
exposure conditions and compared to normoxic mice (NMX) in the right ventricle
(C) and left ventricle (D). No significant differences were found in the right
ventricle/left ventricle ratio (E). The dotted line indicates basal conditions.
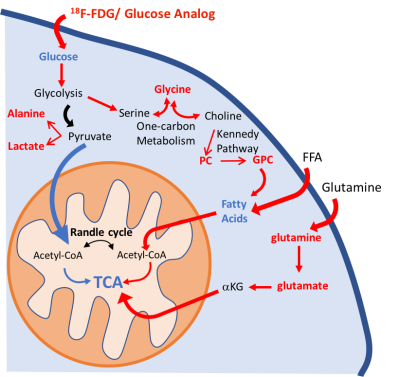
Schematic illustration of the metabolic pathways found altered in the
lung parenchyma. We have identified significant alterations in the glycolytic
pathway: increase in lung 18F-FDG uptake, higher lactate and alanine
concentrations, and a decrease in glucose concentration in PAH mice. Higher
glycine concentration is characteristic of an upregulation of one-carbon
metabolism. Higher levels of phosphocholine (PC) and glycerophosphocholine
(GPC) also point to an increase in lipid biosynthesis. The upregulation of the
glycolytic pathway will reduce metabolic flux into the mitochondria.
Glutaminolysis and beta-oxidation were increased to support TCA activity. Increased
pathways/metabolites are displayed in red, decreased pathways/metabolites in
blue.