3676
Including bone in DIXON-based PET/MRI attenuation correction for primary and recurrent prostate cancer1Department of Circulation and Medical Imaging, NTNU, Norwegian University of Science and Technology, Trondheim, Norway, 2St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway, 3Department of Radiology and Nuclear Medicine, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway, 4Department of Urology, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway, 5Department of Cancer Research and Molecular Medicine, NTNU, Norwegian University of Science and Technology, Trondheim, Norway
Synopsis
Accurate correction for bone attenuation may be important for PET/MRI of prostate cancer due to the high bone-density of the pelvis. In this study we evaluated a previously proposed method that includes bone attenuation coefficients in the DIXON-based attenuation map by co-registration with an atlas of the major bones in the body. We found that the inclusion of bone significantly increased the standardized uptake values of soft tissue lesions, but the effect was only in the order of 3%. In addition, we observed that bone registration errors were present near 31% of the lesions, which may hamper widespread clinical applicability.
Purpose
Attenuation correction is a prerequisite for quantitatively accurate Positron Emission Tomography / Magnetic Resonance Imaging (PET/MRI), but the segmented attenuation maps which are currently used in clinical practice erroneously assign soft-tissue linear attenuation coefficients (LAC) to bone [1]. Paulus et al. recently proposed a method to resolve this issue by including continuous bone LACs in the DIXON-based attenuation map, based on co-registration with an atlas of MR and CT pairs of the major bones in the body [2]. This method may be useful for prostate cancer imaging, where most lesions are found in the bone-dense area of the pelvis and thus underestimations in standardized uptake value (SUV) may be expected. The objective of this study was therefore to evaluate the effect of including bone in DIXON-based attenuation correction for PET/MRI of primary and recurrent prostate cancer.Methods
18F-Fluciclovine PET data from two PET/MRI studies – one for staging of high-risk primary prostate cancer (PPC; 28 patients) and one for diagnosis of recurrent prostate cancer (RPC; 81 patients) – acquired between June 2014 and September 2016 were retrospectively reconstructed with 4-compartment and 5-compartment attenuation maps. All data were acquired on a 3T Biograph mMR scanner (Siemens, Erlangen, Germany) and reconstructed with a vendor-provided algorithm (HDPET, 3 iterations, 21 subsets, 4 mm FWHM Gaussian filter). For the 4-compartment attenuation maps, standard DIXON MR images (TE1/TE2/TR 1.23/2.46/3.6 ms; matrix 192 x 126; coronal slices 128; resolution 4.1 x 2.6 x 3.1 mm3) were automatically segmented into soft-tissue, fat, lung, and (background) air. Fixed LACs were assigned to each compartment [1]. For the 5-compartment attenuation maps, continuous bone LACs were superimposed on the 4-compartment maps by co-registration of the DIXON images with an atlas of MR and CT pairs of the major bones [2]. Vendor-provided software was used for generating both attenuation maps. The 5-compartment attenuation maps were visually compared to the in-phase DIXON images for evaluation of bone registration errors near the lesions. The maximum and 50% isocontour SUVs (SUVmax and SUViso, respectively) were calculated for lesions prospectively scored by a nuclear medicine physician. Linear mixed effects models with patient number as a random effect on the intercept was used to assess the relative change in lesion SUV between the two PET reconstructions (ΔSUV = (SUV5-compartment / SUV4-compartment – 1) x 100%). P-values < 0.05 were considered significant.Results
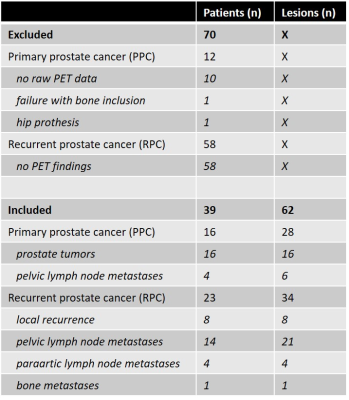
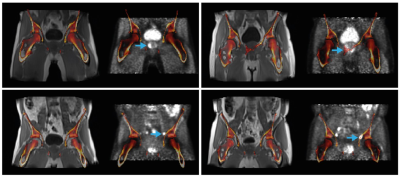
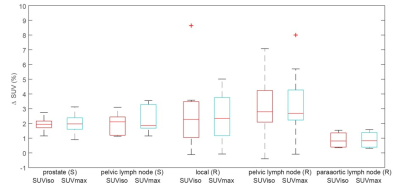
In total, 62 lesions from 39 patients were evaluated (Table 1). Bone registration errors were found near 14/22 (64%) primary tumors (PPC), 1/6 (17%) pelvic lymph node metastases (PPC), 1/8 (13%) locally recurrent tumors (RPC), 2/21 (10%) pelvic lymph node metastases (RPC), 0/4 (0%) paraaortic lymph node metastases (RPC), and 1/1 (100%) bone metastases (RPC). Two examples of bone misregistration are provided in Figure 1. In the remaining 43 lesions without artefacts, the inclusion of bone LACs was associated with a 2.5% (95% CI 2.0%-3.0%; p<0.001) increase in SUVmax and a 2.5% (95% CI 1.9%-3.0%; p<0.001) increase in SUViso in comparison with the 4-compartment attenuation map. The changes in SUV per lesion type are shown in Figure 2.Discussion
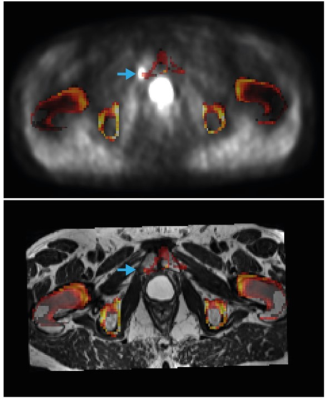
The evaluated method for including bone LACs in MR-based attenuation correction was previously shown to effectively reduce the underestimation in SUV in bone lesions [2] and soft tissue lesions in the brain [3]. To the best of our knowledge, this is the first study to evaluate the method for prostate cancer lesions in the pelvis and lower abdomen. We found small but significant increases in the SUVs of soft-tissue lesions, which were independent of the distance to the closest bone. Although the observed increases in SUV most likely represent reductions in the underestimation of the true SUVs in the bone-dense area of the pelvis, it is questionable how relevant these improvements are in clinical practice. Larger increases in SUV may be expected for pelvic bone lesions than for soft-tissue lesions [2]; the bone lesion detected in our cohort indeed showed 9% increase in SUVmax and SUViso, but registration artefacts were present in this case (Figure 3). In fact, we found bone registration artefacts near 31% of the lesions of our cohort, which may hamper applicability in clinical practice. However, CAIPIRINHA-accelerated DIXON imaging, which provides higher resolution images in the same scan time, could potentially reduce these registration errors [4]. A true reference standard for the SUVs was unfortunately lacking in this study, as CT scans were not acquired.Conclusion
Atlas-based inclusion of bone in 18F-Fluciclovine PET/MRI attenuation correction for primary and recurrent prostate cancer has only a small effect on the SUVs of soft-tissue lesions. The attenuation maps should always be checked for registration artefacts in lesions in or close to bone.Acknowledgements
No acknowledgement found.References
[1] Martinez-Möller A, Souvatzoglou M, Delso G, Bundschuh RA, Chefd'hotel C, Ziegler SI, Navab N, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med, 2009;50(4):520-6
[2] Paulus DH, Quick HH, Geppert C, Fenchel M, Zhan Y, Hermosillo G, Faul D, et al. Whole-Body PET/MR Imaging: Quantitative Evaluation of a Novel Model-Based MR Attenuation Correction Method Including Bone. J Nucl Med, 2015;56(7):1061-6
[3] Koesters T, Friedman KP, Fenchel M, Zhan Y, Hermosillo G, Babb J, Jelescu IO, Faul D, Boada FE, Shepherd TM. Dixon Sequence with Superimposed Model-Based Bone Compartment Provides Highly Accurate PET/MR Attenuation Correction of the Brain. J Nucl Med, 2016;57(6):918-24
[4] Freitag MT, Fenchel M, Bäumer P, Heußer T, Rank CM, Kachelrieß M, Paech D, et al. Improved clinical workflow for simultaneous whole-body PET/MRI using high-resolution CAIPIRINHA-accelerated MR-based attenuation correction. Eur J Radiol, 2017;97:12-20
Figures