3673
Stability of silica coating of magnetic nanoparticles for cell tracking1Institute for Clinical and Experimental Medicine, Prague, Czech Republic, 2Faculty of Mathematics and Physics, Charles University, Prague, Czech Republic, 3Institute of Physics of the Czech Academy of Sciences, Prague, Czech Republic
Synopsis
Magnetic nanoparticles are broadly used for cell tracking in vivo using magnetic resonance imaging. Silica coating, often used for magnetic nanoparticles, represents an inert and stable barrier between the nanoparticle core and cell environment. However, the coating may disintegrate at high temperatures and expose the core. We monitored this process using dynamic relaxometry and confirmed substantial extend of dissolution by transmission electron microscopy. The process probably reflected the equilibrium of solid silica and soluble silicic species in highly dilute suspensions at higher temperature.
Introduction
Magnetic nanoparticles have been used in various medical applications and research for a long time. They could serve as contrast agents for magnetic resonance imaging (MRI) or magnetic particle imaging (MPI), heating mediators for magnetic hyperthermia, and advanced drug delivery systems with magnetically triggered effects. Together with their magnetic core, an integral part of the nanoparticles represents their coating. It should primarily form a stable barrier between the core and the intracellular environment, but may enable functionalization according to desired applications.
Besides (carboxy-) dextran in commercially available nanoparticles, diverse compounds like mannose, poly-L-lysine, hyaluronic acid and other biocompatible materials have been used for coating. However, their long-term stability is questionable and sugar-based coatings are thought to be metabolized1. Another option is represented by an amorphous silica coating, which provides biologically inert surface and stable barrier around magnetic cores with an optional further surface modification2.
We studied silica coating stability at higher temperatures using dynamic MR relaxometry (measuring evolution of relaxivity in time with changing temperature) and transmission electron microscopy.
Methods
The aluminium-doped ε-Fe2O3 nanoparticles of the composition Al0.23Fe1.77O3 were synthesized in mesoporous silica matrix that was subsequently removed, and pure particles were isolated. The particles were further coated with amorphous SiOx(OH)y (silica). The coating and its integrity was analyzed by transmission electron microscopy (TEM). Concentration of nanoparticles in the parent suspension was determined by inductively coupled plasma mass spectrometry (ICP-MS), and the suspension was diluted to 0.6 mM (Al0.23Fe1.77O3) for further measurements.
Relaxometry was performed at 0.47 T (20 MHz) in the temperature range 5 – 80°C. T2 relaxation time in the diluted sample with suspended nanoparticles was at first measured during increasing temperature, then, after reaching 80°C, the measurement continued during its cooling. Sample temperature was measured by a probe inserted directly into the suspension between each two T2 measurements. The sample was shortly mixed before each measurement to avoid sedimentation (in the case of possible precipitation). Relaxivity r2 was calculated as a reciprocal value of T2 related to concentration.
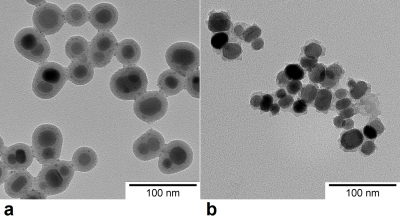
The sample was subjected to TEM analysis after the relaxometry measurement to check the integrity of the nanoparticle coating.
Results and Discussion
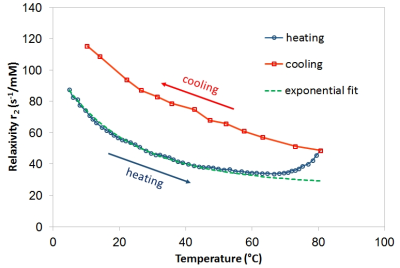
Relaxivity of the nanoparticle suspension decreased with increasing temperature (Fig. 1, blue circles). At lower temperatures, the dependence followed an exponential curve, which means that the relaxivity is probably strongly affected by some 1H transport/exchange processes (such as diffusion in motional averaging regime) with Arrhenius-like dependence on temperature that inversely decrease the relaxation rate. Specifically, the dependence of relaxivity can be described by the relation r2 ≈ (exp(-Ea/RT))-1 where Ea is the activation energy related to the given process, R denotes the gas constant, and T is temperature. However, at temperatures above 50°C, a noticeable deviation from exponential curve occurred (Fig. 1, green dashed line), and above 70°C, even an increase of r2 was observed. The observed effects at high temperatures were in fact irreversible. During cooling of the sample, relaxometry revealed increased r2 in the whole temperature interval (Fig. 1, red squares). Aggregation of the particles was excluded; ultrasound agitation had no influence on relaxivities. Subsequent inspection of the sample by TEM revealed that silica coating was partially dissolved (Fig. 2), which efficiently decreased the distance of the closest approach for water molecules.
The damage of silica shells can be attributed to the newly established equilibrium between solid condensed silica and water-soluble silicic species in highly dilute suspension at higher temperatures, whereby most of the original silica coating was dissolved, and eventually precipitated again as free silica. Although the dissolution process was observed at temperatures substantially exceeding body temperature, it may have important consequences related to particle storage and pre-treatment before in vivo applications.
Conclusion
We revealed a dissolution of silica coating at high temperatures in dilute nanoparticle suspensions using dynamic relaxometry and confirmed the finding by TEM. As the coating represents an important part of the nanoparticles in terms of toxicity, attention should be paid to conditions, which may affect silica coating integrity.Acknowledgements
Grant support: Czech Science Foundation - project No. 16-04340SReferences
1. Novotna B, Turnovcova K, Veverka P et al. The impact of silica encapsulated cobalt zinc ferrite nanoparticles on DNA, lipids and proteins of rat bone marrow mesenchymal stem cells. Nanotoxicology. 2016; 10(6):662-670.
2. Liu, S. and Han, M.-Y. (2010), Silica-Coated Metal Nanoparticles. Chem Asian J, 5: 36–45.
Figures