3671
Improving classifications of brain tumor tissue with Sparse Dictionary Learning based analysis of dynamic susceptibility contrast enhanced MRI data1Emory University School of Medicine, Atlanta, GA, United States, 2The University of Georgia, Athens, China, 3Long Hua Hospital, Shenzhen, China, 4Emory University School of Medicine, Atlanta, China, 5The University of Georgia, Athens, GA, United States
Synopsis
We analyzed the DSC MRI signals based on patterns of descriptive DSE-MR parameters by using Sparse Dictionary Learning (SDL) coding method. We successfully decomposed DSC MRI signals into linear combinations of multiple components based on sparse representation of DSC MRI signals in the tumor region of tumor core and peritumoral edema which might be represent multiple heterogeneity component in brain tumors. Assessment of diagnostic performance of SVM classification after cross validation revealed that the combination of conventional DSC temporal characteristics and dictionary learning based DSC temporal features would result in the best classification accuracy between tumor core and peritumoral edema (with total diagnostic accuracy of 77%, AUC 0.78).
Introduction
Dynamic susceptibility contrast-enhanced MRI (DSC-MRI) is widely applied in studying blood perfusion and vassel permeability in brain tumors. We report a new data-driven and model -free parameterization process by using decomposition-based functional parcellation algorithm of Sparse Dictionary Learning (SDL)2 for analyzing data from DSC MRI of brain tumors. SDL integrates dictionary learning, sparse representation of DSC-MRI time course data, and k-means clustering into one optimization problem, which enables automatically differentiate the different tumor tissue compartments based on the characteristics of the DSC time course profiles. The performance of SDL-based perfusion MRI in characterizing tumor tissue was further evaluated by machine learning algorism.Methods
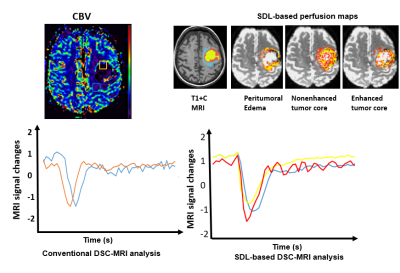
Patients: The DSC MRI data were obtained from patients with high grade glioma (GBM and grade III, n=18) and low grade glioma (Grade II, n=7). MRI Protocol: All patients received MRI scans based on a brain tumor imaging protocol on a 3T MRI scanners (Siemens, Magnetom TrioTim), including pre and post-contrast T1 and T2 weighted imaging, FLAIR and DWI. For DSC MRI, Single-shot gradient-echo (GE) echo-planar imaging was used with TE/TR of 45/2000. Time course data with 70 volumes were recorded 10 seconds after injection of 0.15-mmol/kg bolus of Gd at a rate of 3 mL/s at 60. Imaging analysis: Conventional DSC MRI: Time course data were analyzed using the DSCoMAN plugin (Duke University) in ImageJ to obtain rCBV, rCBF, MTT, TTP maps as described by Boxerman et al 3. Sparse Dictionary Learning (SDL) based DSC-MRI analysis: The computational framework of dictionary learning and sparse coding of DSC MRI signals2 was applied to extract the features of signal time courses in all voxels of selected tumor areas. Then, after normalizing the signals to zero mean and standard deviation of 1, they were arranged into a big signal data matrix X ∈R t×n , where n columns are DSC MRI signals from n voxels and t is the DSC MRI time points. By using aneffective online dictionary learning and sparse coding method4, each DSC MRI signal vector in X is modeled as a linear combination of atoms of a learned basis dictionary D, i.e., Xi = D × αi and X = D × a, where α is the coefficient weight matrix for sparse representation and each column αi is the corresponding coefficient vector for Xi. Classification and cross-validation. We applied support vector machine (SVM) classifier to classify the tumor core and peritumoral edema in either high grade or low grade glioma. Classifiers were trained using the repeated (3 repeat iterations) 10 fold cross validation of training cohort and their predictive performance was evaluated in the validation cohort using area under ROC curve (AUC).Results
SDL-based perfusion analysis successfully decomposed DSC MRI signals into linear combinations of multiple components. With selected dictionaries (or features) derived from the region specific time course data using SDL algorithm, tumors, especially high grade ones, exhibit the regions of enhanced or non-enhanced tumor core and peritumoral edema as shown in Figure 1. The temporal characteristics of the time course data were identified in those corresponding regions. We used classification of tumor core and peritumoral edema as a measure to evaluate the performances of the classification frameworks. The accuracy and the area under the ROC curve (AUC) are presented in table 1. Assessment of diagnostic performance of SVM classification after cross validation revealed that the combination of the conventional and dictionary based analysis of DSC MRI data would result in the best classification accuracy. In high grade gliomas, the diagnostic accuracy is of 80% and AUC of 0.76. In low grade glioma, the diagnostic accuracy is of 70% and AUC of 0.62. By carefully analyzing SVM classification outcome by combination of multiple DSC-MRI features, it can be inferred that employing a more complicated model could refine the improved performance of the classification framework, implying the inherent potential of the DSC-derived descriptive features in differentiation of tumor core and peritumoral edema.Discussion and conclusion
We decomposed DSC MRI signals into linear combinations of multiple components based on Sparse Dictionary Learning Clustering. These perfusion features were validated by using SVM classification to highlight the heterogeneity of the tumor region in either high or low grade tumor. Although preliminary, this method may help characterize malignant tumor regions that would have otherwise not been recognized by using current model based analysis approaches. The results of this study represent the new methods for analyzing the MR perfusion signal that enable improved characterization of the contrast enhanced, non-contrast enhanced tumor region or peritumoral region which may be used to augment targeted therapy, monitoring treatment response and provide patient specific prognostication.Acknowledgements
No acknowledgement found.References
1. Martin, D.R., et al. Individual kidney blood flow measured with contrast-enhanced first-pass perfusion MR imaging. Radiology 246, 241-248 (2008).
2. Lv, J., et al. Holistic atlases of functional networks and interactions reveal reciprocal organizational architecture of cortical function. IEEE Trans Biomed Eng 62, 1120-1131 (2015).
3. Boxerman, J.L., Schmainda, K.M. & Weisskoff, R.M. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27, 859-867 (2006).
4. Mairal, J., Bach, F., Ponce, J. & Sapiro, G. Online Learning for Matrix Factorization and Sparse Coding. J Mach Learn Res 11, 19-60 (2010).
Figures