3669
Rapid and robust high-resolution mapping of proton pool size ratio in spinal cord after injury in squirrel monkeys1Institute of Imaging Science, Vanderbilt University Medical Center, Nashville, TN, United States, 2Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 3Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, United States
Synopsis
High-resolution quantitative magnetization transfer (qMT) MRI provides a noninvasive means to detect and characterize myelination before and after neural injury and during repair. This study aims to systematically evaluate the accuracy and precision of pool size ratio (PSR) measurements using either 5-, 2- or 1-parameter modeling for assessing injury-associated changes in spinal cords in squirrel monkeys in order to optimize a rapid, sensitive, and high-resolution PSR mapping protocol for applications in primates at high field. In addition, the sensitivity of PSR to demyelination in the dorsal pathway rostral and caudal to an injury site has been evaluated.
Introduction
Demyelination is a hallmark of the effects of spinal cord injury (SCI), and quantitative magnetization transfer (qMT) MRI provides a noninvasive means to detect and grade myelination after an injury and during repair. This study aims to systematically evaluate the accuracy and precision of estimates of pool size ratio (PSR) from qMT using different modeling approaches in data analyses for monitoring injury-associated changes in the spinal cords of squirrel monkeys. A primary goal is to optimize a rapid, sensitive and high-resolution PSR mapping protocol for assessing SCI in primates at high field.Methods
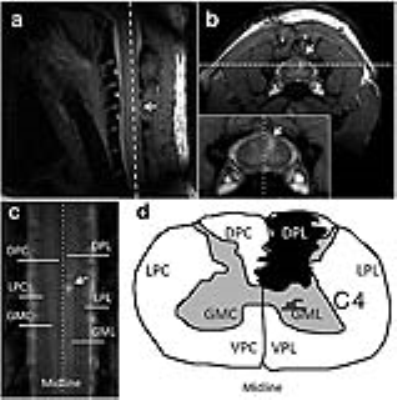
MRI scans were performed in anesthetized squirrel monkeys at 9.4T, before and after a unilateral dorsal column lesion of the cervical spinal cord. Quantitative MT data were collected for a coronal slice (Fig. 1), using a 2D MT-weighted spoiled gradient recalled-echo sequence (TR 24 ms, flip angle = 7°, resolution = ~0.313x0.313x1 mm3, 32 acquisitions). Gaussian-shaped saturation pulses (θsat = 220° and 820°, pulse width = 12 ms) were used with 12 RF offsets spaced at constant logarithmic intervals between 1 and 100 kHz. Histological sections using myelination stains were obtained post mortem for comparison. MRI data were analyzed using MATLAB. A 5-parameter model,1 and 2- and 1-parameter models2 with a reduced number of RF offsets were applied to derive estimates of PSR. 5Pfit derives from 5-parameter fitting using all the qMT data. 2Pfit represents 2-parameter fitting results using only 2-6 RF offsets (defined as 2Pfit(2RF), 2Pfit(3RF), 2Pfit(4RF), 2Pfit(5RF), and 2Pfit(6RF) respectively). Direct 1-parameter measures with different RF offset pairs (one at 100 kHz), are denoted by the other selected RF offset e.g. 820RF4 (~3.5 kHz), 820RF5 (~5.3 kHz), 820RF6 (~8.1 kHz), 820RF7 (~12.3 kHz) and 820RF8 (~18.7 kHz) for data obtained with θsat 820°, and 220RF1 (1 kHz), 220RF2 (~1.5 kHz), 220RF3 (~2.3 kHz), 220RF4 (~3.5 kHz), and 220RF5 (~5.3 kHz) for data using θsat 220°. Numerical simulations were also performed to test modeling performance for different approaches and SNR.3 The regional correlations between PSR from different approaches were calculated using the Pearson correlation function, and it was assumed that 5Pfit was the most accurate method of analysis. The significance of PSR differences was evaluated using Student’s t-tests.Results
The performance of different data analyses was systematically evaluated for the simulated data. Higher SNR and a larger number of RF offsets significantly reduced experimental errors and variance (Fig. 2a). At least 3 RF offsets are required in 2Pfit to retain comparable accuracy and precision as that obtained from 5Pfit with 12 RF offsets (Fig. 2a). The 1-parameter calculation requires higher SNR (more than 1.5 times) to approach comparable accuracy and precision as 5Pfit with 12 RF offsets (Fig. 2a). The 1-parameter calculation is very sensitive to RF offset and power, but the optimum offset and power can be determined. Relative bias in PSR value was estimated for 2-parameter fitting (Fig. 2b). All the selected modeling approaches detected the lesion/cyst as a change in PSR at the site of injury (Fig. 3a). However, the regional contrasts in PSR maps from the different approaches varied, with 2Pfit showing strong positive correlations with 5Pfit (Fig. 3b). The variations from 1-parameter measures were large across RF offsets, powers and sessions at the experimental SNR (~75), and 820RF6 and 220RF3 showed stronger correlations with 5Pfit than measures using other selected RF offsets for 1-parameter modeling (Fig. 3b). Histology confirmed that reduced values of PSR corresponded to demyelination along the dorsal pathway on the injury side (Fig. 4). The rostral side (to the lesion) showed more severe demyelination than the caudal side in the representative injured subjects (Fig. 4). The optimum 2- and 1-parameter approaches were very sensitive in detecting cysts (Fig. 5a), and they also provided comparable sensitivity as 5-parameter modeling to detect regional demyelination and loss of macromolecules around the injury site (Fig. 5a). The decrease of PSR in GM (Fig. 5a) was not as evident as that in the dorsal pathway. The results from 2- or 1-parameter modeling could underestimate PSR of cyst (Fig. 5b), due to the relative bias (Fig. 3b) caused by fixing RaT2a, T2b, and RM0a when their actual values are very different in cyst.4Conclusions
These results support the use of the optimized 2- or 1-parameter approaches for qMT imaging of injured spinal cord as a means to reduce total imaging time and/or permit additional signal averaging. PSR detected demyelination rostral and caudal to lesions, especially in the dorsal pathway on the lesion side.Acknowledgements
We thank Mrs. Chaohui Tang and Mr. Fuxue Xin of the Vanderbilt University Institute of Imaging Science for their assistance in animal preparation and care in MRI data collection. This study is supported by DOD grant W81XWH-17-1-0304, and NIH grants NS092961, NS078680, and NS093669.References
1. Ramani, A., et al., Precise estimate of fundamental in-vivo MT parameters in human brain in clinically feasible times. Magn Reson Imaging, 2002. 20(10): 721-31.
2. Underhill, H.R., et al., Fast bound pool fraction imaging of the in vivo rat brain: Association with myelin content and validation in the C6 glioma model. Neuroimage, 2011. 54(3): 2052-2065.
3. Cercignani, M. and D.C. Alexander, Optimal acquisition schemes for in vivo quantitative magnetization transfer MRI. Magn Reson Med, 2006. 56(4): 803-10.
4. Wang, F., et al., Longitudinal Assessment of Spinal Cord Injuries in Nonhuman Primates with Quantitative Magnetization Transfer. Magn Reson Med, 2016. 75(4): 1685-1696.
Figures