3664
Parameters From Dynamic Contrast-Enhanced Magnetic Resonance Imaging Are Biomarkers Predicting Response after Radiotherapy to Brain Metastases1National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China, 2GE Healthcare, China, Beijing, China
Synopsis
Dynamic contrast-enhanced (DCE) MRI provides additional information regarding blood-brain barrier integrity, and Ktrans is directly proportional to the level of permeability of the blood-brain barrier. In our study, we found demonstrates that SRS of cerebral metastasis is associated with a reduction of Ktrans values in the early post-treatment period. DCE-MRI derived parameters of may be a promising imaging biomarker of tumor aggressiveness.
Introduction
Cerebral metastases are the most common intracranial tumors in adults and occur in approximately 15–25 % of all cancer patients . Stereotactic radiosurgery (SRS) is a non-invasive alternative to surgical resection of brain metastasis. Radiological criteria to evaluate the response of brain metastases to SRS in the early post-treatment period have not been well established. Therefore, an imaging method that could be used to detect early tumor response would be helpful in identifying the true early response of the tumor after SRS treatment.Purpose
Dynamic contrast-enhanced (DCE) MRI provides additional information regarding blood-brain barrier integrity, and Ktrans is directly proportional to the level of permeability of the blood-brain barrier. The purpose of this study was to evaluate the effect of SRS on cerebral metastases with Ktrans that was assessed using DCE MRI. Furthermore, we aimed to evaluate the ability of Ktrans measurements to predict midterm tumor outcomes after SRS.Materials and Methods
All patients underwent a single high-dose SRS performed using a Clinac 6EX photon linear accelerator (Varian, Palo Alto, CA, USA). Seven patients received whole-brain radiation therapy prior to SRS with a mean dose of 3,000 cGy divided into ten fractions. MRI was performed in Optima MR360 (GE, Waukesha, WI, USA). DCE MRI was added to the usual brain tumor protocols using a spoiled gradient-echo acquisition. Fifty dynamic phases were obtained with a temporal sampling interval of 6s and a total imaging time of 6.1 min. The bolus injection of 0.1 mmol/Kg of the contrast agent was administered after the second set of dynamic images at a rate of 2.5 mL/s. DCE MRI data were then processed. Parametric maps were used to measure Ktrans at the regions of interest (ROIs), and the highest (maximum) values were used for analysis, the tumor volumes were subsequently calculated. All statistical analyses were performed with SPSS 19.0, the relationship between Ktrans and tumor volume was assessed using Spearman’s rank correlation coefficient, hazard ratios (HRs) for the association between Ktrans and midterm tumor progression were obtained using Cox proportional hazard models.Results
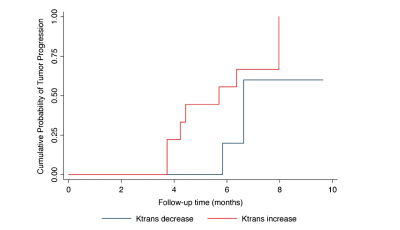
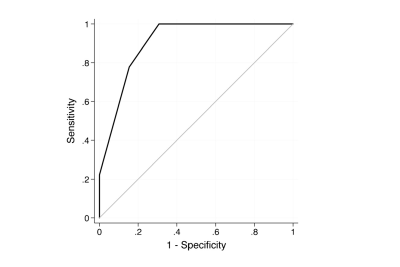
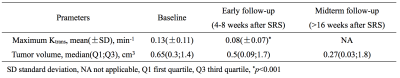
The Ktrans of the metastatic lesions presented a significant reduction after SRS treatment. The mean (±SD) Ktrans value was 0.13±0.11 min−1 at the baseline and 0.08±0.07 min−1 at the early post treatment follow-up (p<0.001) (Figure.1). There was a significant positive correlation between the Ktrans ratio and the tumor growth ratio (Spearman’s coefficient=0.58, p=0.005). The mean baseline Ktrans values were not significantly correlated with the mean midterm tumor volumes. An increase in the Ktrans ratio was significantly associated with lesion progression. Each unit increase in the Ktrans ratio after SRS was associated with an added risk of 50 % of tumor progression (HR=1.50, 95 % CI=1.16–1.70, p<0.001) (Table.1). The Ktrans ratio showed a sensitivity of 78 % and a specificity of 85 % when a cutoff of 0.15 (increase of 15 %) for predicting midterm lesion outcomes (Figure.2).Conclusion
This study demonstrates that SRS of cerebral metastasis is associated with a reduction of Ktrans values in the early post-treatment period. Furthermore, Ktrans variation is predictive of midterm tumor outcome. These results suggest that DCE MRI Ktrans may be a valuable biomarker to evaluate treatment responses in the early post-SRS period during which the identification of treatment failure is more critical. Further large-scale studies may provide a proof of the potential capability of this new method and could also evaluate its performance when combined with other MRI techniques.Acknowledgements
No acknowledgement found.References
1. Rahman M, Cox JB, Chi YY et al. Radiographic response of brain metastasis after linear accelerator radiosurgery. Stereotact Funct Neurosurg 2012; 90:69–78.
2. Kassner A, Thornhill R. Measuring the integrity of the human blood-brain barrier using magnetic resonance imaging. Methods Mol Biol 2011; 686:229–245.
3. Zhang N, Zhang L, Qiu B et al. Correlation of volume transfer coefficient Ktrans with histopathologic grades of gliomas. J Magn Reson Imaging 2012; 36:355–363.
4. Thompson G, Mills SJ, Stivaros SM, Jackson A. Imaging of brain tumors: perfusion/permeability. Neuroimaging Clin N Am 2010; 20:337–353.
5. Peng SL, Chen CF, Liu HL, et al. Analysis of parametric histogram from dynamic contrast-enhanced MRI: application in evaluating brain tumor response to radiotherapy. NMR Biomed 2012.
Figures