3656
Mapping the functional and anatomical signatures of chemogenetically modulated neurons in the salience network.1Singapore Bioimaging Consoritum, Agency for Science, Technology and Research, Singapore, Singapore, 2Singapore Immunology Network, Agency for Science, Technology and Research, Singapore, Singapore
Synopsis
BOLD functional magnetic resonance imaging (fMRI) provides crucial information about the large-scale organisation and function of the healthy and diseased brain. Despite its widespread use, identifying the neuronal-basis for specific functional imaging-based signatures, identified in human disorders and animal models, remains a mostly unmet challenge. Presently, we combine chemogenetic neuromodulation with whole-brain resting-state mouse fMRI and tissue clearing, to reveal both the functional contribution and spatial localization of a targeted neuronal population on resting-state functional connectivity. This approach enables researchers to examine the functional role played by selected neuronal populations on distributed neuronal networks.
Introduction
Resting-state functional connectivity (rs-FC) offers a unique window in the functional macro-organisation of the human and animal brain. Despite its widespread use to characterize the functional signatures in brain disorders and in animal models, a clear translational path to relate these observations to cellular/molecular mechanisms remains mostly lacking. The latter limitations are due to (i) the lack of specificity of the resting BOLD signal and (ii) differences in scales between the rs-FC network described and the underlying neuronal populations forming these networks. Optogenetic or chemogenetic approaches allow to dynamically modulate activity within genetically-defined neuronal populations. When used on animal models, these approaches offer a method to identify the functional signature of targeted neurons onto distributed rs-FC networks, thus overcoming the first limitation. Chemogenetic control is achieved by using “designer receptors exclusively activated by designed drug” (DREADD), a family of proteins leading to either depolarization (hM3Dq neuronal activation) or hyperpolarization (hM4D(Gi) neuronal inhibitor) upon interaction with its ligand clozapine N-oxide (CNO). Moreover, using tissue clearing approaches such as uDISCO [1], it becomes possible to directly relate transfected neurons at a microscopic scale to spatially distributed rs-FC networks, thus overcoming the second limitation. Presently, we have combined both approaches using chemogenetic-controlled neuronal inhibition in order to identify the functional signature of glutamatergic neurons within the insular cortex, a hub region within the salience network and a candidate population affected in depressive disorders [2].Method
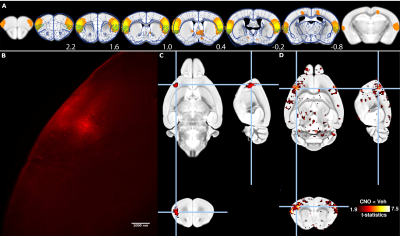
C57B6/J female mice (n=6) were injected stereotactically with AAV8-CaMKII-hM4D(Gi)-mCherry viral vector (Addgene) into the insular cortex (x=3.1mm, y=1.2mm, z=1.5mm, 1ul, Fig. 1A). Animals were imaged 3 weeks post-injections using a resting-state functional magnetic resonance (fMRI) protocol optimized for the mouse [3, 4]. Animals were anesthetized using isoflurane 0.5% and medetomidine 0.1mg/kg/h i.v., and mechanically ventilated under muscle relaxation with pancuronium bromide. Animals were imaged over two sessions, either 20 min following s.c. administration of hM4D(Gi) ligand CNO or vehicle. Images were collected on an 11.75T Biospec equipped with a 2x2 phased-array receiver cryoprobe. Gradient-echo EPI were collected with the following parameters: repetition time (TR) 1000ms, echo time (TE) 15ms, flip angle 50°, 0.18x0.15 mm² in plane resolution, 0.35mm slice thickness, 28 slices, number of volumes 1200. Functional images were processed using the FIX pipeline [4]. Animals were perfused transcardically with cold PFA 4% and processed with uDISCO tissue clearing method[1]. Cleared brains were imaged with light-sheet microscopy (Ultramicroscope, LaVision Botec GmbH, Germany)to reveal mCherry fluorescence. Cleared brains were imaged with MRI using a 3D TurboRARE sequence: TR 1800ms, effective TE 33.9ms, RARE factor 16, number of average 2, 0.1 mm³ resolution. Fluorescence imaging volumes were downsampled and registered linearly to the corresponding ex-vivo MR volumes. Non-linear transformations estimated between the ex-vivo MR volumes and standard AMBMC space (Australia Mouse Brain Mapping Consortium, www.imaging.org.au/AMBMC/AMBMC) were applied to the fluorescence imaging volumes.Results
Rs-FC network overlapping with the insular cortex was identified based on an independent component analysis decomposition carried out previously (Fig. 1A) [4], and it is proposed as the putative rodent salience network [5]. Animals recovered from the surgical procedures without notable changes in animal behaviour. Viral vector injection led to the robust expression of hM4D(Gi)-mCherry fusion protein within identifiable cell bodies as imaged with light-sheet microscopy (Fig. 1B). Registration of a unilaterally-injected ex-vivo brain to AMBMC space reveals a fluorescent focus overlapping with the insular cortex (Fig. 1C). Tissue-clearing induced deformations could be effectively accounted for during the normalization process (Fig. 2). Administration of CNO, the ligand for inhibitory DREADD hM4D(Gi), lead to widely reduced rs-FC within the targeted resting-state functional network (Fig. 1c), compared to vehicle administration.Discussion
Relating rs-FC signatures to specific neuronal events remains a difficult conceptual barrier to breach, due to differences in scales and the indirect nature of the haemodynamic BOLD signal recorded with fMRI. Combining the neuromodulatory possibilities of chemogenetic, whole-brain functional imaging, and high-resolution whole-brain fluorescent imaging, allows for mapping of the distal modulatory effect that selected neuronal populations exert on distributed resting-state networks. This combined approach is expected to validate neuronal populations identified in animal models of brain disorders, thus guiding therapeutic efforts toward imaging-based targets identified in human disorders and animal models.Acknowledgements
No acknowledgement found.References
1. Pan, C., et al., Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat Methods, 2016. 13(10): p. 859-67. 2. Grandjean, J., et al., Chronic psychosocial stress in mice leads to changes in brain functional connectivity and metabolite levels comparable to human depression. Neuroimage, 2016. 3. Grandjean, J., et al., Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage, 2014. 102 Pt 2: p. 838-47. 4. Zerbi, V., et al., Mapping the mouse brain with rs-fMRI: An optimized pipeline for functional network identification. Neuroimage, 2015. 123: p. 11-21. 5. Gozzi, A. and A.J. Schwarz, Large-scale functional connectivity networks in the rodent brain. Neuroimage, 2016. 127: p. 496-509.Figures