3652
Rapamycin treatment increases cerebral blood flow and attenuates anxiety in pre-symptomatic APOE4 mice: effects of sex and APP transgene1Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY, United States, 2Pharmacology and Nutritional Sciences, University of Kentucky, Lexington, KY, United States, 3Harvard University, Cambridge, MA, United States, 4Biomedical Engineering, University of Kentucky, Lexington, KY, United States
Synopsis
APOE4 is the strongest genetic risk factor for Alzheimer’s disease (AD). Pre-symptomatic APOE4 carriers have developed neurovascular deficits decades before amyloid beta (Aβ) aggregation. Here we show that with Rapamycin treatment for 16 weeks, pre-symptomatic APOE4 mice had restored cerebral blood flow (CBF) and attenuated anxiety, compared to those of APOE3 mice. The CBF restorations were particularly significant in female mice or those with APP transgene. As Rapamycin and MRI and are readily to be used in humans, the findings may provide valuable information for future clinical trials to prevent AD for APOE4 carriers.
Introduction
The ε4 allele of Apolipoprotein E gene (APOE4) is the strongest genetic risk factor for Alzheimer’s disease (AD)1. A hallmark of AD is amyloid beta (Aβ) aggregation, however Aβ detected too late in the aging process. Human neuroimaging studies have indicated that APOE4 carriers develop vascular deficiency several decades prior to Aβ deposition, which further leads to neuronal loss, anxiety and dementia2,3. In this study we investigated effect of Rapamycin (Rapa), a FDA approved drug, on genetically modified AD mice with Human APOE3 and APOE4 alleles, as a preventative therapeutic for AD4. We hypothesize that Rapa is able to preserve cerebral blood flow (CBF) in pre-symptomatic APOE4 mice, which is associated with reduced anxiety of the mice. We also hypothesized that there will be different sex and APP transgene (Aβ) effects on CBF in response to Rapa.Methods
Mice were with APOE gene, with or without expressing 5 familial-AD (5xFAD) APP mutation. We fed APOE3 and APOE4 mice with either control vehicle or Rapa diet (low dose; 14 ppm) from 3 months of age and continued for 16 weeks. We have four groups animals, with N = 12 each group (F:M= 6:6). MRI procedure: MRI images were acquired using a 7T Clinscan MR scanner (Siemens, Germany). Quantitative cerebral blood flow (CBF) images were acquired using a pseudo-continues arterial spin labeling (pcASL) technique at both pre- and post-treatment time points. The following parameters were applied: field of view read (FoV) = 18.0mm, FoV phase = 75.0%, slice thickness = 1mm, 6 slices, 120 measurements, base resolution = 64, phase resolution = 100%, repetition time (TR) = 4000ms, echo time (TE) = 200us, label offset = 17mm, post-label delay = 0 us, 200 RF blocks, RF GAP = 200 us, RF duration = 200us, maximum Gz = 90mT/m, mean Gzx10 = 70, and phi adjust = phi = 95-105. Behavioral assessment: We used elevated plus maze (EPM) to assess anxiety level of the mice at the end of the study. The maze consists of four arms (two enclosed arms and two open arms) elevated 100 cm above the floor (Fig. 3A). The time that mouse spent in the closed arms and open arms of the maze were recorded automatically over the 5 min test session by EthoVision XT 8.0 video tracking software (Noldus Information Technology). Statistical analyses: For CBF, changes between pre- and post- treatment will be calculated. EPM had the post-treatment measurement. Data analysis will employ a two-way ANOVA (Treatment x Genotype) to establish differences in Rapa treatment effects on imaging and biochemical outcomes, followed by Tukey’s post hoc test.Results
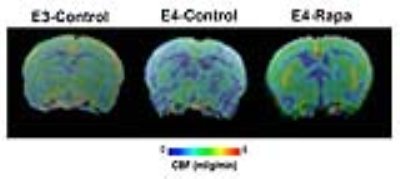
Figure 1 shows the MRI-based CBF images, showing that pre-symptomatic E4-Control had significantly lower CBF compared to E3-Control. However, with Rapa treatment, APOE4 mice (E4-Rapa) had restored CBF to the level similar of E3-Control. We further looked into APP transgene effects on CBF; we found Rapa treatment significantly increased CBF in the APP (+) group (p = 0.017), regardless of APOE genotype, but not in the APP (-) group (Figure 2A). Female mice showed a significant increase in CBF after Rapa treatment (p = 0.027), whereas the effects were not significant in males (Figure 2B). In the EPM test (Figure 3A), we found that E4-Control mice spent significantly less time in the open arm, indicating higher anxiety, compared to the E3-Cotrol mice (p < 0.01). With Rapa treatment, however, APOE4 mice had undistinguished anxiety level compared to APOE3 mice (Figure 3B).Discussion
Rapamycin is a FDA-approved drug, which has been used as an immunosuppressant in patients with organ transplant. However, using low dose (< 0.5 therapeutic range), Rapa has shown to increase lifespan in various species5 and enhance immune functions in older adults with minimal side effects6.Here we showed that using low dose of Rapa, pre-symptomatic APOE4 mice had restored CBF and reduced anxiety levels to levels similar to APOE3 mice. The effects were particularly significant in groups with higher risk for AD, such as APP (+) and female mice.Conclusion
Our results show the potential of Rapa as an effective intervention to prevent AD for pre-symptomatic APOE4 carriers. As Rapa, MRI and behavioral assessments are readily to be used in humans, the findings from the study may provide valuable information, and may pave a way, for future Rapamycin clinical trials to prevent dementia among pre-symptomatic APOE4 carriers.Acknowledgements
The study is supported by NIH/NIA K01AG040164, NIH/NIA R01AG054459, and NIH/CTSA UL1TR000117 to Ai-Ling Lin.References
1. Liu, C.C., et al., Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol, 2013. 9: 106-18.
2. Bangen, K.J., et al., APOE genotype modifies the relationship between midlife vascular risk factors and later cognitive decline. J Stroke Cerebrovasc Dis, 2013. 22: 1361-9.
3. Bell, R.D., et al., Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature, 2012. 485: 512-6.
4. Lin, A.L., et al., Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer's disease. J Cereb Blood Flow Metab, 2013. 33: 1412-21.
5. Harrison, D.E., et al., Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 2009. 460: 392-5.
6. Mannick, J.B., et al., mTOR inhibition improves immune function in the elderly. Sci Transl Med, 2014. 6: 268ra179.
Figures