3646
Multi-modal multi-centre MRI study of rare spinocerebellar ataxia, type 141Institute of Neuroscience and Medicine 4, Forschungszentrum Jülich, Jülich, Germany, 2Department of Neurology, Faculty of Medicine, RWTH Aachen University, Aachen, Germany, 3Institute of Neuroscience and Medicine 1, Forschungszentrum Jülich, Jülich, Germany, 4Department of Neurology, University Hospital Bonn, Bonn, Germany, 5NeuroCure Clinical Research Center, Charité – Universitätsmedizin Berlin, Berlin, Germany, 6Department of Psychiatry and Psychotherapy, University Hospital Bonn, Bonn, Germany, 7Department of Neurology, Charité – Universitätsmedizin Berlin, Berlin, Germany, 8Department of Neurology, University of Duisburg-Essen, Essen, Germany, 9Department of Neurodegenerative Diseases, Hertie-Institute for Clinical Brain Research, University of Tübingen, Tübingen, Germany, 10German Research Center for Neurodegenerative Diseases (DZNE), University of Tübingen, Tübingen, Germany, 11Institute of Medical Genetics and Applied Genomics, University of Tübingen, Tübingen, Germany, 12Center of Stroke Research Berlin (CSB), Charité - University Medicine Berlin, Berlin, Germany, 13German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 14C. and O. Vogt Institute for Brain Research, Heinrich Heine University Düsseldorf, Düsseldorf, Germany, 15Center for Movement Disorders and Neuromodulation, Department of Neurology and Institute of Clinical Neuroscience and Medical Psychology, Medical Faculty, Heinrich-Heine University, Düsseldorf, Germany
Synopsis
Diffusion MRI studies reveal significant white matter degeneration in cerebellar and cerebral regions of spinocerebellar ataxia patients, however, only common pathology types have been included. Here, we present the first combined deformation-based morphometry (to depict regions of volume loss), and diffusion tensor/
Introduction
Spinocerebellar ataxias (SCA)1,2 constitute a genetically heterogeneous group of neurodegenerative movement disorders characterised by progressive imbalance (ataxia), ophthalmoplegia, peripheral neuropathy, cognitive dysfunction, and dementia. The pathological changes in the SCAs consist of degeneration of cerebellar structures and of several other brainstem or deep brain nuclei as well as cortical regions, depending on the SCA subtype. The rare autosomal dominant SCA of type 14 (SCA14) is a late onset progressive disease caused by mutations in protein kinase C gamma (PKCγ). Since its genetic definition in 2003, it is increasingly recognized among patients with hitherto undefined SCAs in Germany. Patients mostly present with a slowly progressive cerebellar ataxia, but additional symptoms (cognitive decline, hyper-reflexivity, myoclonus, dystonia) have been reported that might reflect extracerebellar (EC) damage. Case series reported mild to severe cerebellar midline atrophy and sometimes additional pontine, brainstem or cerebral atrophy. Previous diffusion tensor imaging (DTI) studies3,4 of some more commonly studied SCA types have revealed significant degeneration of white matter (WM) tracts in cerebellar and cerebral regions, while increases in diffusivity and decreases in anisotropy were correlated with clinical scores. However, no DTI examinations have been thus far reported for SCA14. This multimodal MRI study was therefore designed to elucidate the pattern of brain structural alterations with combined MRI, including deformation-based morphometry (DBM) to depict subtle volume loss, and diffusion kurtosis imaging (DKI)5, a novel extension of DTI, to analyse microstructural changes in the largest reported SCA14 sample enrolled in a coordinated multi-centre study. In particular, we focus on the following: Can DTI/DKI metrics provide an evidence for EC alterations in WM? Are structural and microstructural indices correlated with clinical scores, such as disease duration and severity scores?Materials and Methods
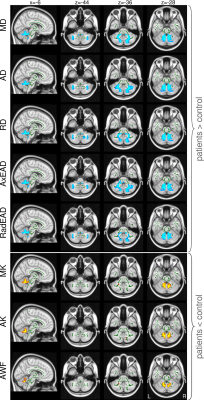
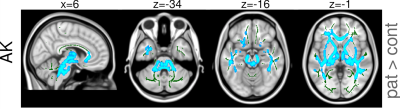
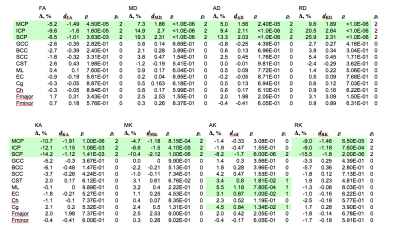
Clinically well-characterized SCA14-patients (DBM(N=22, 9 m): age 49.4±12.9 years, disease duration 16.7±12.8 years; DTI/DKI(N=20, 10 m): age 48.4±11.5 years, disease duration 15.6±12.0 years) and matched healthy controls underwent MRI (3T MAGNETOM Trio; Siemens). Imaging protocol comprised MPRAGE (voxel-size: 1×1×1 mm³) and diffusion-weighted sequences (60 gradient directions, b-values 0, 1000 and 2500 s/mm², voxel-size: 2×2×2 mm³, matrix-size: 128×116×70). We performed between-group comparisons of DTI/DKI parameters (fractional anisotropy (FA), kurtosis anisotropy (KA), mean/axial/radial diffusivity (MD/AD/RD), mean/axial/radial diffusion kurtosis (MK/AK/RK)) and, additionally, axonal water fraction (AWF) and extra-axonal axial/radial diffusivity (AxEAD/RadEAD) from the WM model6 using voxel-based and a region of interest (ROI)-based approaches. (DBM with p<0.001, DTI/DKI using tract-based spatial statistics (TBSS) with ptfce<0.05, and p<0.05 for ROI-based analysis). In ROI-based analysis, between-group differences were quantified as relative percentual changes (Δ) and Cohen’s d values evaluated for the regional parameter means.Results
Group differences in most parameters including volume reduction in DBM and changes in DTI/DKI metrics were predominantly restricted to the cerebellum and cerebellar peduncles (see Figures 1 and 2 for the results of the TBSS analysis of diffusion metrics and Table 1 for the results of the ROI analysis). ROI analysis revealed large significant between-group differences in superior, middle, and inferior cerebellar peduncles for all DTI/DKI diffusion parameters (except for AK and RK) with the largest Δ (up to ca. 26% in SCP) and Cohen’s d values (up to 2.64) provided by RD. In EC regions, volume loss was present along the medial lemniscus only, accompanied by increased AK and AxEAD. Among DTI/DKI metrics, only AK revealed between-group differences in EC regions, showing an extensive increase in SCA14 patients localized within pontine crossing tract, corticospinal tracts, external and internal capsules, callosal genu, fornix, superior/inferior longitudinal and occipitofrontal fasciculus. Moderate correlations (r between 0.46 and 0.6, p<0.05) were found in a few EC regions (but in none of cerebellar peduncles) between several DTI/DKI metrics and clinical scores of disease duration and severity.Discussion and Conclusions
This is the first multi-modal imaging study of the rare SCA14 pathology. Cerebellar volume deficits are in line with the reported high expression of PKCγ in Purkinje cells. Volume reduction and diffusivity changes detected along cerebellar peduncles suggest that not only efferent fibres but also afferent fibers are affected. Sensory deficits reported by SCA14 patients may be related to (micro-)structural changes along medial lemniscus. Additionally, higher AK outside the cerebellum in the absence of volume reduction may indicate impaired axonal coherence in axial direction. EC symptoms (dystonia/myoclonus) were present in our SCA14 sample and can be potentially related to these microstructural changes, suggesting that SCA14 pathology could be associated with systematic global WM alterations. Alternatively, the impaired EC axonal coherence might reflect disturbed cerebello-cortical circuits or even a development of potential compensatory mechanisms, and requires deeper investigation in future.Acknowledgements
No acknowledgement found.References
[1] Paulson HL. The Spinocerebellar Ataxias, J. Neuroophthalmol., 2009, 29:227-237.
[2] Manto M, Habas C. Cerebellar disorders: clinical/radiologic findings and modern imaging tools. Handb Clin Neurol., 2016;135:479-91.
[3] Mandelli ML, De Simone T, Minati L, et al. Diffusion tensor imaging of spinocerebellar ataxias types 1 and 2, AJNR Am. J. Neuroradiol., 2007, 28:1996-2000.
[4] Hernandez-Castillo CR, Galvez V, Mercadillo R, et al. Extensive White Matter Alterations and Its Correlations with Ataxia Severity in SCA 2 Patients, PlosOne, 2015, 10: e0135449.
[5] Jensen JH, Helpern JA, Ramani A, et al. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med., 2005, 53:1432-1440.
[6] Fieremans E, Jensen JH, Helpern JA. White matter characterization with diffusional kurtosis imaging. Neuroimage, 2011, 58:177-188.
Figures