3643
Deep Learning on Anatomical Brain MRI to Classify Motor Dysfunction in Parkinson's Disease1Institute of Biomedical Engineering, National Taiwan University, Taipei City, Taiwan, 2Interdisciplinary Institute of Neuroscience and Technology, Qiushi Academy for Advanced Studies, Zhejiang University, Hangzhou City, China, 3The PhD Program for Neural Regenerative Medicine, Taipei Medical University, Taipei City, Taiwan, 4Department of neurology, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou City, China, 5Department of Biomedical Engineering, National Yang Ming University, Taipei City, Taiwan
Synopsis
We introduced an innovative two-staged deep artificial neural network (DNN) model focusing on diagnostic prediction of Parkinson’s disease (PD) using T1-weighted images, given a training set consisting of cortical thickness, surface area, grey matter volume and corresponding clinical scales, our proposed model was trained to classify the PD with different motor symptoms and performed the diagnostic prediction on basis of generated clinical scales. Results showed our DNN classifier and generator reached the averaged accuracy of 100% and 97.9%, respectively. To our knowledge, our technique was the first to tackle the classification of motor dysfunction in PD from anatomical brain MRI.
Introduction
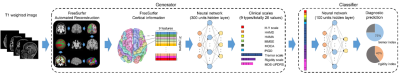
Clinical Decision Support (CDS) systems assisted clinicians in detection and interpretation of diseases. In the past, CDS systems were confined to marking and evaluating the conspicuous regions in medical images, such as detection of breast cancer with mammograms1 or nodules in thoracic CT2. Some CDS systems had developed a fully-automated computational solution and focused on prediction of certain diseases using machine learning tools such as artificial neural network3 (ANN) or support vector machine4 (SVM). However, since the ambiguity of parameters in the machine learning technologies, the decision logic might be unexplainable, and thus those algorithms never met the need of clinical application. To deal with above-mentioned problems, we proposed a CDS model based on the deep artificial neural network (DNN) automatically learn hierarchies of relevant features directly from the raw inputs of cortical thickness, surface area and grey matter volume to identify unique morphological patterns of cortical atrophy in Parkinson’s disease (PD) and to relate these patterns of change to disease duration and clinical features. Our CDS model was composed of two components: 1) A generator which extracts information from medical images and generates explainable clinical scales. 2) A classifier which evaluates clinical scales from generator and makes the final diagnostic prediction. We train our model on Parkinson’s disease (PD) using T1-weighted images (T1WIs). The training and validation process involved patients with Parkinson’s disease (PD) and the healthy control subjects aged 43 to 69 years old. As the result, the classifier achieved 100% average accuracy on both training set and validation set using 3-fold cross-validation; we then evaluated generator using the well-trained classifier, and the generated clinical scales achieved 99.5% average accuracy as well.Methods
Subjects:
All patients (N = 28) and the healthy control (N =19) subjects were examined by neurologists with more than twelve years of experience in movement disorders. Age at onset, disease duration and severity of motor symptoms, as assessed by the Unified Parkinson’s Disease Rating Scale (UPDRS) and postural instability gait difficulty (PIGD), and the Hoehn–Yahr (H&Y) stage, were recorded. The Hamilton Depression Scale (Ham-D), Hospital Anxiety and Depression Scale (HADS), Mini Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA) were used to assess general cognitive status. The group of 28 PD patients consisted of 21 patients only with the rigidity symptom, 5 patients only with the tremor symptom, and 2 patients with the symptoms of rigidity and tremor.
Training Data and Deep Learning Model:
For all subjects, we had a set of training data each one consisting of 1) T1WIs of whole brain: 9 indices of 31 cortical areas, totally 279 values, were calculated using FreeSurfe5. These 9 indices comprised the information of cortical thickness, mass and curvature. 2) 9 different clinical rating scales: UPDRS (5 features), PIGD (1 feature), H&Y scale (1 feature), Ham-D (1 feature), Ham-A (1 feature), MMSE (1 feature), MoCA (1 feature), tremor scale (9 features), and rigidity scale (6 features). Totally 26 features were involved. Our model was a variation of encoder-decoder model6. Fig. 1 showed the model consisted of two independent end-to-end neural network, generator and classifier. Generator was composed of FreeSurfer6 preprocessing and a two-layered neural network fed by cortical information (279 features) and clinical scales (26 features). The main function of generator was to interpret T1WIs and generate satisfactory prediction of clinical scales. The classifier was also a two-layered neural network. In training stage, classifier was fed by true clinical scales and the type of subjects, learning to make preferable diagnostic prediction. Outputs of classifier contained two indices ranged 0 to 1, representing the possibility of being tremor type and rigidity type respectively. If both indices were lower than 0.5, we considered the subject as a healthy one. At last, the well-trained classifier was used to evaluate the performance of generator. The whole architecture was implemented by Tensorflow software7. The training procedure lasted 20,000 epochs, updated using stochastic gradient descent. To improve our model, we applied dropout8 mechanism to both generator and classifier.
Results and Discussion
We evaluated our model using 3-fold cross-validation. Classifier reached 100% average accuracy on both training set and validation set. The performance of generator was evaluated based on prediction of well-trained classifier, and reached 97.9%, 98.9% average accuracy on validation set and training set respectively (Table 1).
Conclusion
We introduced a brand new CDS model which not only made diagnostic prediction but also generated explainable clinical scales for further diagnosis. Furthermore, our model reached 97.9% average accuracy on identifying PD patients with different motor symptoms (rigidity or tremor)