3641
Cerebellum and Sensory Motor Resting State Network Behaviors in tremor- and akinetic rigid -predominant patients with Parkinson’s disease1Radiology, Drum Tower Hospital, Medical School of Nanjing University, Nanjing, Nanjing, China, 2Radiology, Center for NMR Research, Penn State University College of Medicine, Hershey, PA, United States, 3Neurology, Penn State University College of Medicine, Hershey, PA, United States
Synopsis
The striatal dopamine depletion in Parkinson's disease (PD) explains clinical symptoms such as bradykinesia and rigidity, but not resting tremor thought to be associated with cerebellothalamic (CTC) circuit dysfunction. In this study we used resting state fMRI to investigate differences in the CTC network in akinetic-rigid (PDAR) and tremor predominant (PDT) PD patients with closely matched demographic and cognitive variables. The results support a type dependent functional disruption in the cerebellum and SMA rs-networks in PD patients with otherwise comparable disease severity, neurocognitive performance, and overall brain morphology.
Abstract
INTRODUCTION: Parkinson's disease (PD) is traditionally characterized by tremor, rigidity, and bradykinesia [1, 2, 3, 4, 5]. Following the most prominent motor symptoms, two major PD subtypes have been identified: akinetic-rigid (PDAR) and tremor predominant (PDT). Recent evidence suggests that PDT may have different underlying pathophysiology when compared to PDAR [1]. For example, the cerebello-thalamo-cortical (CTC) pathways are thought to be impaired differentially in PDT [1]. Despite these observations, very little is known about the pathophysiological changes of the CTC network in PD. This study employed resting-state fMRI (rs-fMRI) to investigate differences in the CTC network in PDAR and PDT samples with closely matched demographic and cognitive variables. Since most task-related brain network behaviors are recapitulated during resting state, we hypothesized that differential resting state behaviors are detectable in the sensory motor and cerebellum networks in PDAR and PDT [6, 7].
METHODS: We studied 17 PDAR and 15 PDT participants with similar disease severity, and 24 healthy controls (HC). Study samples were matched in age, gender, disease severity, and cognitive performance (based on comprehensive neuropsychological tests). The rs-fMRI data was acquired on a Siemens 3T MRI system (Magnetom Trio, Siemens Medical, USA) using an 8-channel head coil with the following parameters: TR / TE / FA = 2000 ms / 30 ms / 90°; FOV = 240 ´ 240 mm2; acquisition matrix = 80 ´ 80; # of slices = 34; slice thickness = 4 mm, and the number of repetitions = 240. A 3D MPRAGE image was also acquired for volumetric analysis and anatomical overlay. Subject order-independent group independent component analysis (SOI-GICA) was used for the ICA decomposition [8]. A voxel-wise functional connectivity (FC) analysis of resting state data was performed using the dpabi software [9].
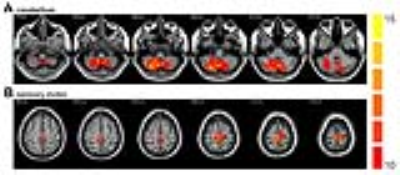
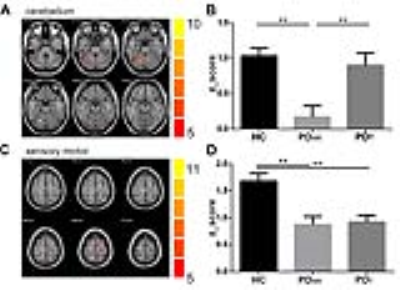
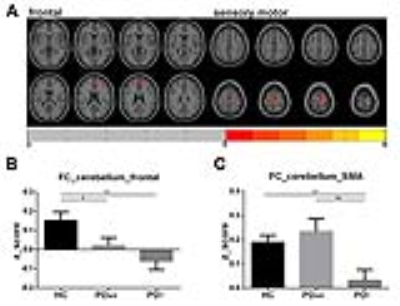
RESULTS: The group ICA method reliably identified the sensory motor and the cerebellum networks in all three groups (Figure 1). Group differences among HC, PDAR and PDT in respective networks are illustrated in Figure 2. Significantly lower activation was detected in the right cerebellum in PDAR. In the SMA network, both PDAR and PDT samples showed significantly lower activation than HC. The region that showed group differences in the cerebellum was related to a FC pattern that was markedly different between PDAR and PDT (Figure 3). A VBM (voxel-based morphometry) analysis revealed no differences among the three groups at a family-wise corrected p-value of p <0.05.
CONCLUSION: Results support a type dependent functional disruption in the cerebellum and SMA rs-networks in PD patients with otherwise comparable disease severity, neurocognitive performance, and overall brain morphology. Our findings shed new insight into the critical roles played by the CTC network in the pathophysiology of PDT and PDAR. Specifically, cerebellar activation in PDAR was markedly reduced along with functional connectivity to the frontal cortex. In contrast, PDT do not have reduced activation but rather markedly reduced functional connectivity between cerebellum and frontal cortical and SMA regions [1, 2]. Thus, rs-fMRI should be further investigated to evaluate its usefulness as a possible diagnostic tool (or biomarker) capable of identifying PD patients with different patterns of functional pathophysiology and potential outcomes. Future studies may provide insights into the role of cerebellum and its related neural networks subserving both akinetic-rigid and resting tremor predominant PD.
Acknowledgements
This work was supported by the NINDS grant NS060722, the HMC GCRC (NIH M01RR10732), GCRC Construction Grants(C06RR016499), and the Department of Radiology, Pennsylvania State University College of Medicine. The authors report no conflict of interest.References
[1]. Caligiore, D. (2016), ‘Parkinson’s disease as a system-level disorder’, npj Parkinson's Disease, vol 2, 16025; doi:10.1038/npjparkd.2016.25
[2]. Karunanayaka, P. (2016), ’Default mode network differences between rigidity- and tremor-predominant Parkinson's disease’, Cortex, vol 81, pp 239-250.
[3]. Aarsland, D. (2010), ‘The epidemiology of dementia associated with Parkinson disease’, J Neurol Sci, vol 289, no 1-2, pp 18-22.
[4]. Lewis, M.M. (2011), ‘Differential involvement of striato- and cerebello-thalamo-cortical pathways in tremor- and akinetic/rigid-predominant Parkinson's disease’. Neuroscience, vol 177, pp 230-239.
[5]. Helmich, R.C. (2012), ‘Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits?’, Brain, vol 135, no 11, pp 3206-3226.
[6]. Deco, G. (2011), ‘The dynamical balance of the brain at rest’, Neuroscientist, vol 1, pp 107-23, doi: 10.1177/1073858409354384. Epub 2010 Dec 31.
[7]. Greicius, M.D. (2003), ‘Functional connectivity in the resting brain: a network analysis of the default mode hypothesis’, Proceedings of the National Academy of Sciences of the United States of America, vol 100, no 1, pp 253-258.
[8]. Zhang, H. (2010), ‘Subject order-independent group ICA (SOI-GICA) for functional MRI data analysis’, NeuroImage,vol 51, no 4, pp 1414-1424.
[9]. Yan, C.-G. (2016), ‘DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging’. Neuroinformatics: pp 1-13.
Figures